Journal of
eISSN: 2373-437X


Research Article Volume 4 Issue 2
1Department of Pathological analysis, Thi-Qar University, Iraq
2Department of Microbiology, Al-Nahrain University, Iraq
3Head of Biology Department, Cihan University, Erbil, Iraq
Correspondence: Ahmed H AL Khafaji, Pathological analysis Department, College of Science, Thi-Qar University, Nasiriya, Iraq, Tel 964 7801949172
Received: July 15, 2016 | Published: March 1, 2017
Citation: AL Khafaji AH, AL Jeboori TI, Juma ASM (2017) Study the Role of Some Types of T Lymphocytes in Toxoplasmosis. J Microbiol Exp 4(2): 00107. DOI: 10.15406/jmen.2017.04.00107
Toxoplasma gondii has become increasingly recognized as an important pathogen of humans and domestic animals. It is one of the most common causes of latent infections in humans over the world. Venous blood was collected from the patients and controls, A total of sixty women with a history of abortion and twenty-four healthy-looking controls were included in this study. Forty-four women were found to have Toxoplasma infection, fourteen women with anti-Toxoplasma IgM antibody (acute infection), eighteen with anti- Toxoplasma IgG antibody (past or chronic infection), and twelve with both IgG and IgM antibodies (recurrent infection). CD3, CD4, CD8, and CD71 were used and the results showed a significant elevation of these markers in women with toxoplasmosis when compared to the healthy controls. Toxoplasmosis cause both acute and chronic infections leading to stimulate T lymphocytes that provide protection against multiple infections and prevent occurrence of complications.
Keywords: toxoplasmosis, abortion, CD markers
CD, cluster of differentiation
Toxoplasmosis
Toxoplasma gondii was first discovered in an African desert rodent in 1908. It was then found in many species of mammals and birds worldwide and also in humans, but not until recently has it been realized that it can cause human disease. There are many Toxoplasma species in animals but only a single species is capable of causing human disease. T. gondii has been maintained in a colony at the Pasteur institute in Tunis. Since then, the parasite has been found in almost every country of the world in many species of carnivores, insectivores, rodents, pigs, herbivores, primates and other mammals as well as in birds. The importance of the organism as a human pathogen has stimulated a huge amount of research in recent years. Since the mid 1980s T. gondii has joined a number of other parasites recognized as a complicating factor for immuno suppressed patients.1
T. gondiiis an obligate intracellular parasite and its life cycle includes both sexual and asexual modes of proliferation and transmission. The sexual cycle takes place exclusively in the intestinal enterocytes of many members of the cat family (Felidae). After ingestion of tissue cysts, the parasites invade the enterocytes, undergo several rounds of division and differentiate into micro gametocytes and macro gametocytes. The gametocytes fuse to form a zygote or “oocyst” that is shed into the environment with the cat’s feces. The oocyst undergoes meiosis, producing an oocyst of highly infectious “sporozoites” that are resistant to environmental damage and may persist for years in a moist environment. After ingestion by a secondary host (such as man), sporozoites differentiate into the rapidly dividing tachyzoite form, which establishes and sustains the acute infection.2
T. gondiiis an important water- or food-born pathogen that is capable of causing severe disease in infants from infected mothers and in the immunocompromised patients.3 It is here that the parasite causes the most severe pathology; disseminated congenital infections in the developing fetus,4 severe neurological complications in immunocompromised individuals5 and ocular pathology in otherwise healthy individuals.6 Within the vertebrate host,Toxoplasma is capable of infecting all types of nucleated cells and of crossing several important cellular barriers. The parasite subsequently penetrates intestinal epithelial cells, and either develops within them, or exits through the basolateral side and burrows across the basement membrane. This parasite invades mammalian cells by an active actin-dependent mechanism, and after entry establishes a vacuole with the assistance of products secreted by the parasite’s apical organelles. Simultaneously, Toxoplasma sets about gaining access to cellular nutrients by forming pores in the vacuolar membrane. In this manner it enjoys a rich and comfortable lifestyle at the host cell expense.7
Role of T-cells
Toxoplasma infection rapidly overcomes hosts with impaired T-cell function and diminished ability to produce the cytokines. In acute infection, the main pathologic findings associated with acute toxoplasmosis are lymphoadenopathy and fever, which occur simultaneously with parasite-induced activation of the immune system and concurrent release of high levels of proinflammatory cytokines. In most patients, acute toxoplasmosis is benign and will evolve to the asymptomatic stage within a few weeks of infection.8 Nevertheless, congenital transmission of Toxoplasma, occurring when a pregnant female is undergoing the acute phase of a primary infection, can result in severe disease in the infant. Briefly, the pathological consequences for the fetus are dependent upon the trimester during which transmission occurs and will vary in severity from mild ocular disease to death.9
Thus, several lines of evidence suggest that Toxoplasma induces inflammatory responses that can contribute to the pathological findings associated with acute infection. T-lymphocytes, in particular the CD4 + subset, as well as granulocytes, contribute to the response. In some circumstances, the parasite is capable of triggering a catastrophic inflammatory cytokine storm, which the animals cannot survive.
In contrast to acute disease, most of the pathologic findings associated with chronic toxoplasmosis in humans are thought to be caused by lack of appropriate T-cell immunity rather than an excessively vigorous response.8 Depletion of T-cells in the setting of chronic infection rapidly leads to reactivation of infection and death.10
Generation of T-lymphocytes possessing parasite-specific cytolytic activity is characteristic of both human and murine infection. In humans, both CD4+ and CD8+ , cells with cytolytic activity have been isolated from seropositive donors. The ability of these T-cell subsets to simultaneously produce the protective cytokine IFN- γ and exhibit cytolytic function has obscured the issue of whether the latter activity plays a role during infection. Nevertheless the finding that CD8+ but not CD4+ T cells are more efficient at transferring immunity has led to the suggestion that CTL activity contributes to protection.8
Patients
Sixty women who had abortion were selected for this study. They were referred to the Central Health Laboratory in Baghdad according to the physician’s reports, indicating the possibility of them having toxoplasmosis. Serum and venous heparinized blood was collected from those women in a period between January 2003 and March 2003. Those women had been divided into three groups according to the presence or absence of specific anti-Toxoplasma antibodies. These groups included; the women with IgG,11 the women with IgM12 and the women with both IgG and IgM.13 The other 16 women had no antibodies against Toxoplasma.
Controls
Twenty-four healthy-looking women had been selected as controls to compare with our patients in the same parameters of this study. Serum and venous heprinized blood was collected from those women and tested for anti-Toxoplasma antibodies. Those that revealed any antibody titer against Toxoplasma were excluded from the study.
Immunostaining of lymphocytes
Isolation of peripheral blood lymphocytes:
Reagents
components were then added:
The final volume was brought to 1000ml with D.D.W., the contents mixed well and filtered through a 0.2μm. Millipore filters (Nagle) and then stored at -20°C until use.
The following contents were dissolved in DW, bringing the final volume to 100ml.
The following contents were mixed together:
It was stored at room temperature until use.
Isolation procedure
Determination of lymphocyte phenotype
Indirect immunostaining
Tris-buffered saline (TBS):
Tris base powder was dissolved in DW at a concentration of 0.5M (60.6g/L). The pH was adjusted to 7.6 with 1 N HCl. The solution was stored at 4°C.
Chamber slides pre-coated with lymphocytes, were removed from freezer, allowed to reach room temperature, unwrapped and washed with PBS. Then, lymphocytes were reacted with different CD markers. 10μl of 1:50 dilution of CD3, CD4, CD8 were left over each spot (each one over each spot) for 90 min at 37 °C with shaking. Then spots were washed 3 times with PBS, to discard unbound antibodies. After washing, spots were reacted with secondary antibody (anti-mouse peroxidase conjugate antibody). 10μl of 1:100 dilution of this antibody were reacted with spots for 1 hour at 37°C with shaking. Then, cells were washed as previously described.
Ten ml of freshly prepared DAB solution, was applied to each spot for about 15-20 minutes or until brown discoloration of the spot appears. Cells were then washed with PBS and stained with hematoxylin for about 1.5 seconds. After staining, spots were rinsed with tap water and then PBS was added for 5 minutes. Mounting was then done for the spots using glycerin. Counting of cells was performed using light microscope (ALTAY) using high power lens (x40 magnification). Their dark brown staining identified the positively labeled cells, while the negative cells were stained with hematoxylin showing blue color (Figure 1). In each spot, 100 cells were counted with differentiation depending on morphology of cells and labeling. This will give the percent of cells with each CD marker.
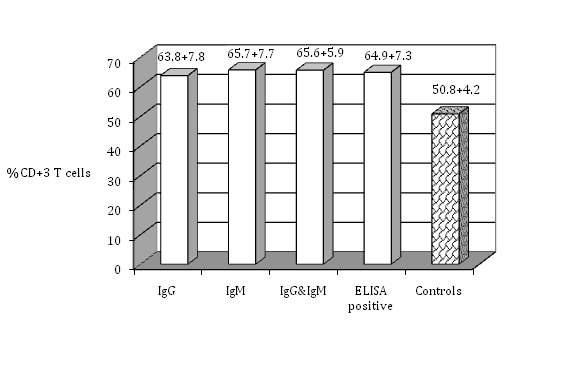
Figure 1 The percentage of CD+3 T cells in the peripheral blood lymphocytes of seropositive women for toxoplasmosis compared with the healthy controls (no antibodies).
Direct Immunostaining
The fixed cells on the slides were the solid cellular antigens to which the fluorochrome-labeled monoclonal antibodies are directed and then by fluorescent microscope we could detect the fluorescently stained cells, indicating that these specific antigens were detected by those monoclonal antibodies.13 Fixed cells on special immunofluorscence slides. Monoclonal fluorescent-labeled antibody CD71: It is the transferrin receptor; it has been expressed on T-lymphocytes, macrophages and monocytes surfaces.16 The Expression of the transferrin receptor protein was examined by Western analysis and found to be specifically elevated in Toxoplasma-infected fibroblasts.12
Chamber slides, pre-coated with lymphocytes, were removed from freezer, allowed to reach room temperature, unwrapped and washed with PBS. 10μl of 1.5 dilution of CD71 were applied to a duplicate spots, and then incubated within a humid chamber at 37°C in the dark shaking for two hours. The spots were then washed and 1 to 2 drops of mounting fluid were added onto each well. The cover slips were placed on the mounting fluid- spotted slides. The slides were examined by fluorescent microscope (Olympus) using 40 x magnification lenses immediately or up to 3 days later as a maximal duration. A suitable countable field is located and the numbers of stained cells were counted. 200 cells were recovered and counted and then the calculation was made as follows:
Percentage of positively stained cells = (number of labeled cells)/ (Total number of cells) x100
Statistical analysis
The data were statistically analyzed using student's T test according to Snedecor and Cochran17 by using
Excel 2003 software.
Ethical approval
This research underwent to the terms of ethical considerations and in accordance with the form prepared for this purpose by the Iraqi Ministry of Health also got the approval of the research by the Committee of ethical standards in the Faculty of Medicine, Al-Nahrain University, one of the colleges affiliated to the Ministry of Higher Education and Scientific Research, Iraq.
The isolated PBL were tested for cell surface markers using monoclonal antibodies against T-cell subsets. The percentage of total T cell (CD3-positive cells) indicates that seropositive women had a significantly higher percent of T cell (63.8±7.8 %) (P≤0.05) than seronegative ones (50.8±4.2%). There was no significant difference among the groups that had IgG, IgM, or both IgG and IgM in their serum samples (P>0.05) (Figure 1). Regarding T-cell subsets; T-helper or T-inducer and T-cytotoxic or T-suppressor (CD4 positive cells and CD8 positive cells respectively), (Figure 2) shows that CD4 positive cells were significantly higher in percent in seropositive women (66.5±6.9%) (p≤0.05) than seronegative ones. There was no significant difference (P>0.05) among the groups that had IgG, IgM, or both IgG and IgM. CD8 positive cells showed significantly increasing number in seropositive women (58.7±4.9%) (P≤ 0.05) than seronegative women, while there was no significant differences among the women who had IgG, IgM, or both IgG and IgM (Figure 3). Seropositive women showed significantly higher percent of CD+71 T cells (63.3±6.7%) (P ≤ 0.05) than those seronegative ones, while there was no significant differences among the women who had IgG, IgM, or both IgG and IgM (Figure 4). There are certain techniques to measure the expressed antigen on activated lymphocytes like rapid flow cytometric method which is relatively accurate and is a rapid technique.18 Measurement of expressed specific activation antigens by Immunostaining method is another technique although it is not as accurate and rapid as the flow cytometric method but in this study it was used for its availability. These activated antigens (CD markers) included CD3, CD4, CD8, and CD71. The results obtained previously indicate that CD3 is the antigen that is expressed by all mature T cells. CD4 is presented on T-helper/T-inducer cells (30% to 60% of all peripheral human T lymphocytes), CD8 expressed by cytotoxic T cells as well as suppressor T cells (15% to 40% of all peripheral human T lymphocytes).15 CD71 is expressed on T-lymphocytes, macrophages and monocytes surfaces.16
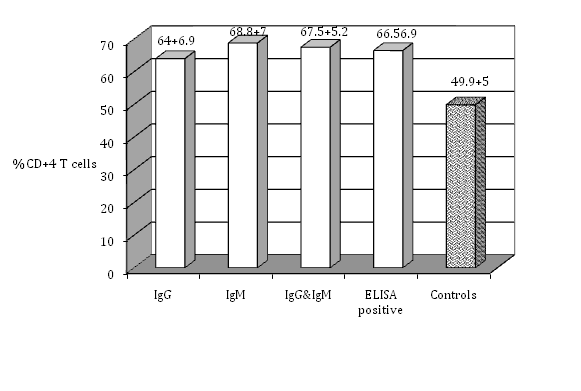
Figure 2 The percentage of CD+4 T cells in the peripheral blood lymphocytes of seropositive women for toxoplasmosis compared with the healthy controls (no antibodies).
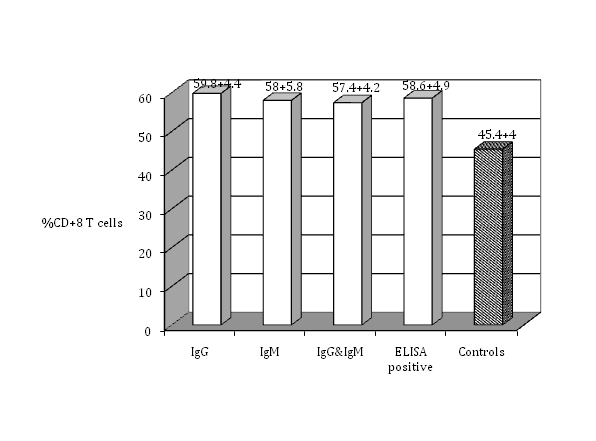
Figure 3 The percentage of CD+8 T cells in the peripheral blood lymphocytes of seropositive women for toxoplasmosis compared with the healthy controls (no antibodies).

Figure 4 The percentage of CD+71 T cells in the peripheral blood lymphocytes of seropositive women for toxoplasmosis compared with the healthy controls (no antibodies).
This study showed a significant increase in the CD+3 T cell of women infected with T. gondii than of the healthy controls. This indicate the strong proliferation of the lymphocyte as a result of exposure to the Toxplasma antigen as published by Kahi et al.18 CD+4 and CD+8 T cell percentage were significantly higher in the women infected with T. gondii than of healthy controls. This may indicate the CTL activity against T. gondii as published by other studies.11 Caruso et al.19 considered the CD71 as a specific marker to T. gondii expressed on the activated T cells when exposed to T. gondii antigen. This study measures the percentage of activated CD+71 T cell in the infected women and the results showed a high significant percentage in this marker of those women when compared to the healthy controls. Both CD+4 and CD+8 CTL are part of the human immune response to T.gondii infection. Both antigen-pulsed and Toxoplasma infected antigen presenting cell (APC) induced cell proliferation. Toxoplasma infected APC elicited strong CD+4-mediated than CD+8- mediated cell proliferation and generated CD+4 CTL more readily than CD+8 CTL.11 CD+71 was regularly induced on a much larger percentage of T lymphocytes from T. gondii seropositive than T. gondii seronegative subjects. This antigen also shows an amplified expression in response to tetanus toxoid or influenza virus antigen and it appears suitable for the evaluation of cellular response.19 These tests proved to correlate well with serological status, and samples from seronegative individuals usually showed no stimulation.18
Conclusion Toxoplasma gondii infection elicit both humoral and cellular immune response, regarding to T lymphocytes, this infection stimulate two types of T cell , CD 4+ cells for acute infection and CD8+ cell for chronic infection in addition to that, this parasite use CD71+ marker on T cell for facilitate its penetration and proliferation.
I grateful acknowledge the support of the team workers in the Microbiology Department in the College of Medicine of AlNahrain University.
Ahmed H AL Khafajy: Preparation, techniques and publication. Tarik I AL Jeboori: Design the plane. Ameena S M Juma: Design the proposal.
The authors declare no conflict of interest.
None.

©2017 AL, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.