Journal of
eISSN: 2373-437X


Review Article Volume 7 Issue 4
Department of Microbiology, Aurora’s Degree and PG College, India
Correspondence: Vasudevan Ranganathan, Department of Microbiology, Aurora’s Degree and PG College (Affiliated to Osmania University), India, Tel 8121119692
Received: June 09, 2019 | Published: July 17, 2019
Citation: Ranganathan V, Akhila CH. Streptococcus mutans: has it become prime perpetrator for oral manifestations? J Microbiol Exp. 2019;7(4):207-213. DOI: 10.15406/jmen.2019.07.00261
Human beings have indeed served as an incubator for a plethora of microorganisms and the prominence of oral microbiome from the context of the individual’s health and well being cannot be denied. The environmental parameters and other affiliated physical conditions decide the fate of the microorganism and one of the niches in humans that supports innumerable amount of microorganisms is the oral cavity which houses beneficial and pathogenic microorganisms. However, majority of microorganism associated with humans are opportunistic pathogens which are otherwise referred to as facultative pathogens. This level of transformation in the microorganism depends upon the physical conditions of the oral cavity and personal hygiene maintained by the individual. The contemporary review tries to disclose the role of streptococcus mutans in dental clinical conditions. The current review focuses on the prominence of Streptococcus mutans and its influence on the oral cavity. The article attempts to comprehend the role of the bacteria in causing clinical oral manifestations which depends upon the ability of the organism to utilize the substrate. The review also encompasses features like molecular entities and they role in the breakdown of the substrates leading to the formation of acids which could in turn lead to demineralization which as a consequence can negatively influence the enamel quality.
Keywords: Streptococcus mutans, dental Biofilms, microbial interactions, oral microflora
The oral cavity of humans and animals is a perfect ecological niche for a range of microbial agents and some of these are capable of inflicting severe clinical conditions. These clinical conditions can lead to manifestations which could escort dire consequences. It could in fact be claimed that the major vicinity of the oral facet has been dominated by several microorganisms. Niches like teeth, gingival sulcus, tongue, cheeks, hard and soft palates, and tonsils are prime spots for the microbes to reside. In fact these areas are dominated by certain species of bacteria and one of the prime contenders that invade these oral areas is Streptococcus species. Among these species, S. mutans are widely regarded as one of the most dominant agent.1,2 Several demonstrative attempts and scientific studies have validated that the oral cavity harbors a plethora of microbial agents of many Streptococci species and it is undeniable fact that many of these species share some common facets. These features could range from their habitat to their feeding habits and their basic mode of survival.3
Studies have also revealed that these similarities among the species could pose a threat in the identification and characterization. However a range of biochemical analysis plays a vital role in comprehending the organism and studies have also employed the genetic and molecular level analysis for accurate flawless characterization. The prominence of biochemical test cannot be refuted as it provides a better understanding in terms of sugar fermentation ability of the organism and also endows the biochemical nature of the organism.3 One group of Streptococci that is commonly affiliated with oral niche is S. mutans and various studies have confirmed the association of this organism with humans. In fact the human oral eco system encompasses a highly diverse oral microbial biota and twenty five different types of Streptococci have been found to inhabit the oral cavity of humans. They represent twenty percent of total oral bacteria and can be hospitable or hostile. Each of these species is capable exhibiting properties that endow them with abilities to inhabit different oral sites and also enable them to compete against the other oral microflora.
Studies also reveal that the bacteria have developed properties that allow them to overcome host factors like immune system, physico-chemical shocks, and mechanical frictions. However further evidences are needed to substantiate these claims. Unsteadiness in the native microbial flora leads to oral conditions resulting in clinical manifestations under appropriate conditions. Under certain conditions the commensal nature of the organism gets transformed to hostile nature which is associated with oral diseases. Hence, they are labeled as opportunistic pathogens that initiate disease and inflict damage to the host. The group of mutans streptococci was regarded as the most important etiological agent leading to the formation of clinical condition called dental caries. Despite the fact that the bacteria are natural resident of the oral flora, they are associated with carious lesions.4
Resident microbes have evolved and coexisted with humans for millions of years which are usually considered as a symbiotic relationship. It would be difficult to sequester ourselves from our microbiome and we are not distinct. The oral microbiome plays a vital role in the physiology and health. It is believed that humans harbor over 700 species of bacteria that are capable of colonizing the hard surface of the teeth and soft areas of the mucous epithelial cells. Novel technologies employed by scientific investigators have indeed deciphered the intricate facets of the oral microbiome and have provided prominent insights affiliated to health and disease. Agitations of the oral ecological niche can lead to dire consequences because it will have an impact on the normal equilibrium of microflora which could in turn hike up the population of undesirable microorganisms capable of clinical manifestations. The modern day life has been stated as a major reason’s that has opened the doors for microorganisms to influence the oral ecosystem of an individual. Infections such as dental cries, gum inflammation and periodontitis have become common due to alteration of oral backdrop favoring the undesirable microorganisms.5
Oral microflora is the common term given to the microorganism found in the human oral cavity. However, terms like oral microbiota or oral microbiome have been recently used to refer these organisms affiliated with the oral cavity.6 The term microbiome is in fact a recently introduced term and is often used from the context of human microbiome project. Several scientific investigators believed that this would enhance the understanding of human health and diseases. Researchers consider the oral microbiome to be a vital aspect which explores oral flora and provides a better scope of identification and characterization of these organisms. It is also believed that this information also serves as a prerequisite in understanding the phylogeny of the oral flora. It is estimated that the oral cavity harbors several hundred species and almost half of these can be cultivated under anaerobic condition in labs.7 Molecular studies have also been employed to comprehend the microorganism and the commonly used analysis is the 16S r RNA gene based studies which involve the cloning of the 16S r RNA. The molecular methods are highly sensitive ad accurate and will provide a strong validation from the context of identification and characterization. The oral cavity encompasses several areas like gums, teeth, tongue, palates and is in fact the major gateway of the human system. Various clinical manifestations as a consequence of infectious disease have been like to oral microflora that is capable of invading the teeth, gums and root canal.8
In fact many microorganisms colonize the human oral ecosystem and it is estimated that an average human system comprises of a microbial population of as much as ten folds.9,10 Some of them are commensal which will coexist with our system with causing any harm and other studies have revealed the existence of mutualism with humans.11,12 However, the significance of the oral ecosystem cannot be denied because the alteration in the normal condition will hamper the normal microbial population which could in turn influence the balance of the oral ecosystem creating conditions favorable for undesirable microorganism to cause dire oral consequences leading to oral clinical manifestations.
About Streptococcus mutans
The bacterium is a common affiliate of the human oral niche and is facultative anaerobic, Gram positive cocci. It is very often related to oral manifestation and is widely regarded as a significant contributor of tooth decay. The existence of this organism came in t lime light when it was described by J. Kilian Clarke in 1924.14 It is believed that this organism can coexist with Streptococcus sobrinus and their collaborative effort leads to a range of oral clinical conditions. The same kind of coexistence can also be seen in case of Streptococcus viridians.15 As exclaimed by several demonstrative studies S. mutans is natural contender of the oral ecosystem in addition to 25 other species that inhabit the oral cavity. However, the process of classification and characterization that are associated with the taxonomy are still speculative.16 In simple terms the taxonomy is yet to be made cohesive. It is a widely accepted fact that different areas of the oral cavity are extremely contrasting and have a range of ecological niche which in turn decide the fate of microorganism in terms of their survival rates. Each of these species has specific properties which allow them to claim over an area in the oral ecological niche. Scientific investigators though their demonstrative studies have validated the dominance of S. mutans on the depressions of the tooth surface otherwise called as pits and studies have proclaimed the presence of these organisms in the grooves of the biting surface which is also called as fissures. This in turn constitute to 39% of streptococci in the oral ecosystem. Apart from these claims, studies also confirm the presence of Streptococcus mutans in the alimentary canal which in turn opens in to pharynx and esophagus which constitute to 2-9%.17 Some studies also substantiate the existence of a symbiotic relationship among bacteria and fungi which results in bacterial fungal co-aggregation. This symbiotic relationship enhances the ability of cariogenic potentiality of S. mutans. A symbiotic relationship between S. mutans and Candida albicans has been authenticated by several scientific demonstrators which lead to elevated production of glucan which in turn is directly proportional to the extent of biofilm formation. It is also believed that this aspect boosts the carcinogenic effect of S. mutans.18 However, the presence of harmful and harmless counterparts of Streptococci in the oral cavity cannot be denied. Therefore, they are referred as opportunistic pathogens which occur under certain conditions which transforms commensal streptococci in to a pathogenic one which could be an indication of the onset of the oral manifestations (Figure 1).
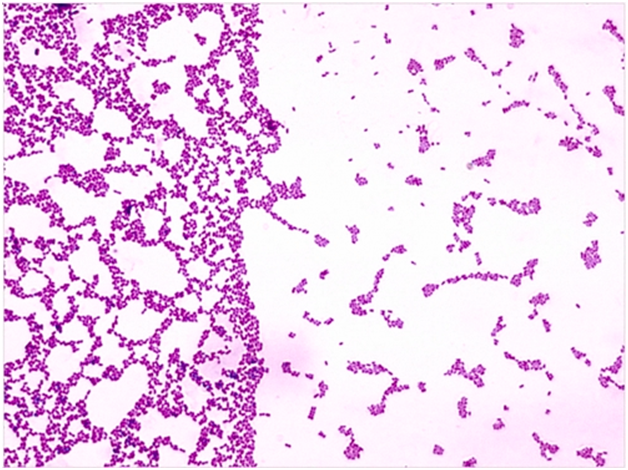
Figure 1 Microscopic depiction of streptococcus mutans.13
It is indeed a widely accepted fact that the ability of the organism to inhabit and invade various areas of the oral cavity makes it a prime perpetrator of tooth decay which can in turn have a deep seated impact on the human oral cavity and the health of the individual.19 The organism is mesophillic and temperatures ranging from 18-400C are appropriate. The temperature within this range is more convenient for the organism to survive and S. mutans is a cariogenic microorganism that is capable of breaking down sugar for its energy requirement resulting in the production of acids. This in turn leads to an acidic environment which might deleterious to the teeth as they have the ability to demineralize the teeth. The acidic environment produced as a consequence of sugar breakdown due to microbial action disintegrates the teeth leading to the dissolving of calcium creating a hole. Individuals of all age groups are vulnerable but are more common in infants and children.
Some studies synchronize the vertical transmission as a major factor where mother can transmit the organism from her to the infant. The transfer of genotypes during child birth from mother to infant is also cited as a major factor that leads to S. mutans infection. Studies also reveal the existence of variations among different populations.20 Healthy individuals is known to harbor 10000 CFU of S. mutans per ml in their oral cavity on an approximate basis.21 The virulent factors present in the organism fortify the organism in terms of diseases causing abilities. The three main virulent factors that are often cited from the context of the organism are water insoluble glycans, acid tolerance and lactic acid production.22 The most commonly experienced symptom of tooth decay is toothache which is a consequence of the tooth pulp irritation leading to toothache. X rays can be used to comprehend the extent of damage caused on the tooth before they transform in to cavities. The easiest way to prevent these dire oral consequences is to lessen the amount of acid fermentation in the mouth. Since the bacteria is a part of natural flora of the oral cavity and is a facultative pathogen as it turn out to be pathogenic under certain condition, is also referred to as opportunistic pathogen and appropriate measure are inevitable to avoid oral clinical manifestations.
Pathogenesis
The pathogenesis from the context of a disease involves progressive biological mechanism which enhances the advancement of the clinical manifestation encompassing a range of morphological features ending up in a diseased state. Based on the extent of impact imposed on the individual, it can be termed as acute, chronic or recurrent.23 In view of the fact that S. mutans is naturally affiliated with the oral niche, it is capable of causing a range of oral condition that is a consequence of complicated biological correspondence. It is a widely accepted fact that S. mutans are known for their biofilm forming abilities and this phenomenon makes them much more stringent and difficult to tackle. These microbial biofilms in fact serve as microscopic rims which offer protection to the microbial population enclosed within the rim. Some studies reveal that the disease causing characteristic also depends on the extent of communication between these opportunistic pathogens which is a very intricate network and is poorly understood.24 Microbial biofilms indeed is highly complicated and involves a series of morphological and biochemical changes during the course of transformation from a planktonic form to a biofilm producer. These biological films are complex amalgamation organic substance and are in fact a polysaccharide matrix which enables the microorganisms to anchor on a platform which could be biotic or abiotic.25
Stages of dental biofilms
(Figure 2)
Transmission of the clinical condition
Dental manifestations as a consequence of S. mutans are a common phenomenon and can progress in to severe outcomes if not treated. In fact dental caries are infectious and transmissible dental manifestations can infect individuals of all age groups. Studies also claim that S. mutans can be passed on among individuals through horizontal or vertical transmission. The organism initially colonize and tries to acclimatize in the human host which is then followed by the onset of the infection which occurs over a period of time and severe cases can also lead to the infection of the gums and tongue. Though individuals are all age group are infected the most vulnerable targets are infant and children and studies claim that this is a consequence of vertical transmission. The infection is common in infants because the non shedding surfaces favor the organism to establish permanent colonies and the presence of the organism will be undetected till primary teeth extravagate.26 Recent studies also claim that the organism is known to inhabit the furrows of the tongue in infants which usually occurs during the teething process. This usually occurs at the age when infant develop one or two teeth which allows the organism to colonize and cause cavities. This could be the indication of cavity formation as a consequence of detectable levels of S. mutans on teeth. Presence of the organism on the furrows of the tongue implies the transmission of the organism vertically from mother to infant which occurs shortly after birth.
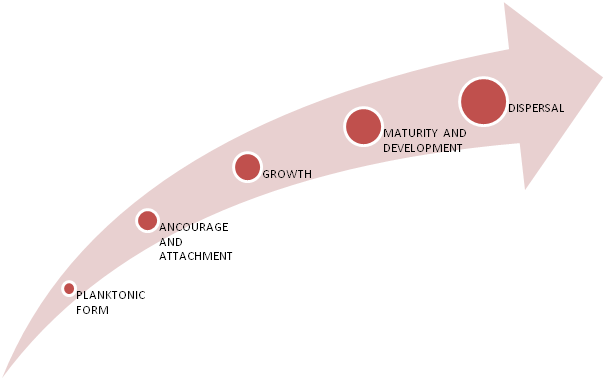
Figure 2 Schematic representation of different stages of bacterial plaque biofilm formation.25
Studies also claim that mothers with history of dental manifestation are at higher scope of transferring the condition to the infants that are likely to possess same levels of virulence. Mothers with salivary samples with a count of over 105 CFU are known to transmit the condition to the infants which is found to be nine time greater when compared to normal circumstances (Figure 3 & 4).
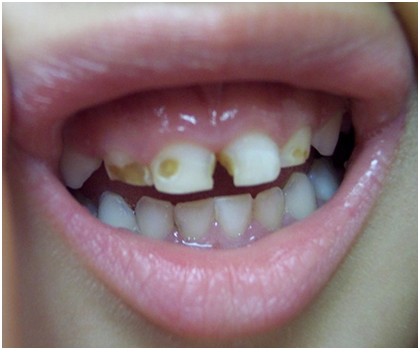
Figure 3 The above image depicts advanced tooth decay in a child.28
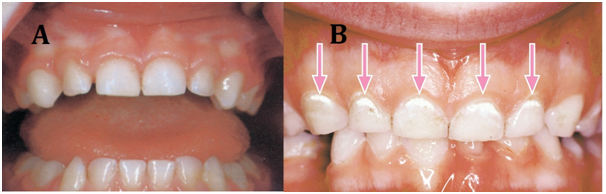
Figure 4 Image a depicts healthy teeth and gums and image b displays the early signs of oral clinical manifestation.28
Colonization
Colonization is a vital process which is one of the steps employed by the organism to complete it manifestation course. S. mutans is known for its ability of altering the environmental conditions of the oral flora during its growth and metabolism which in turn enhances the fastidious nature promoting the colonization of the organism leading to dental plaques. The organism is equipped with specific receptors which promote its attachment to the surface of the teeth and this can be referred as the onset of dental biofilm formation. Following attachment, the organism begins to produce microbial colonies within the slimy biofilm network. S. mutans growth and development results in the formation of dextran with the assistance of dextransucrase which binds to the enamel content of the teeth. This enzyme plays a vital role in the metabolism of sucrose resulting in the formation of glucose and fructose. The organism ferments fructose and polymerizes glucose in to an extracellular dextran polymer. This polymer in turn affixes the organism to the enamel resulting in the formation of intricate biological network. The organism is also capable of de polymerizing glucose for its utilization as a carbon source leading to the production of lactic acid which further decalcifies the enamel resulting in dental caries. The advanced stage of this condition causes tooth decay due to the combination of acid and plaque.29
Cell surface components are vital
Demonstrative attempts to comprehend the adherence ability have revealed various features affiliated with the organism responsible for its anchorage on to a surface. According to earlier studies that were carried out for cohesive understanding of this mechanism, it was believed that the salivary molecules were vital for the attachment to the oral surface resulting in dental caries. However, certain cell surface molecules including proteins and other organic substances have a prominent role in attachment. Studies have revealed that proteins and lipoteichoic acid served in accomplishing adherence of the organism under lab conditions. When human cells were cultured under in vitro conditions these molecules played a significant role in attachment of the organism to these cultured cells. Studies have also highlighted the role fibronectin in anchorage which is a essential protein of the extracellular matrix (Figure 5 & 6).
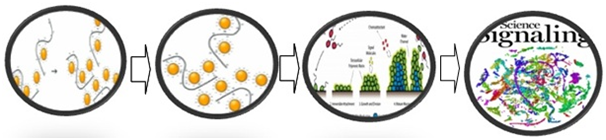
Figure 5 Diagrammatic representation of stages involved in anchorage mechanism.31
Steps involved in anchorage mechanism
The above diagrammatic depiction provides the sequential occurrences of various stages which allow the establishment of the organism. Various components associated with the host and the pathogen enhances the process of attachment which includes the salivary elements of individual and the chemicals secreted by the organism. The process initially starts with the process of preliminary attachment which slowly progresses in to a bridging of high specificity and affinity. This in turn triggers the process of microbial communication through the process of quorum sensing resulting in cell signaling. All these intricate processes play a significant role in the establishment of the microorganism which indeed is vital for the onset of infection.32
Approaches to target streptococcus mutans
It is a widely accepted fact that Streptococcus mutans resides in dental biofilms which could sometimes be an amalgamation of diverse species. Favorable environmental conditions are vital for the organism to rapidly produce acids by fermenting dietary carbohydrates. This in turn leads to demineralization of tooth causing tooth decay.33,34 It is therefore affiliated with oral manifestations and S. mutans is commonly regarded as one of the prime perpetrators leading to dental caries.35 Studies have also revealed the prominence of S. mutans in causing sub acute endocarditis.36 Nevertheless, studies have stated and validated that dental caries is not life claiming and can be diagnosed on time for treatment which is possible by exterminating the undesirable microorganism. The eradication of S. mutans using wide range antimicrobial drug was not advisable because it would also have an impact on the resident microflora leading to imbalance of the oral ecosystem which could in turn lead to clinical and allergic consequences. Hence, there was a need for a novel strategy that could eliminate the undesirable microbe with influencing the normal residents of the oral ecosystem. Therefore, prevention of S. mutans and its affiliated manifestations stipulated the requirement of alternative methods that could specifically target the undesirable organism without interrupting the resident microflora within the oral biofilm.
The recent times has witnessed several attempts to ensure target specific antimicrobial therapy rather than pragmatic measures. The target specific antimicrobial therapy was achieved by the combination of the antibiotics that are species specific. This has also opened the doors for the engineering of species specific monoclonal antibodies or by developing fusion peptides that recognize the bacterial binding domains.37 These novel attempts have in turn lead to the formation of narrow spectrum antimicrobials. Due to their narrow range in terms of efficacy, they are highly selective on undesirable microorganism without a significant impact on the resident microflora. Pheromone guided antimicrobial peptides (PG-AMPs) have been greatly used from the context of selectivity because of its narrow spectrum which depend on targeted killing of the undesirable microorganism.38
Significance of GTF
Several demonstrative studies have also revealed the prominence of oleic acid and the role of glucosyl transferase. In fact, glucosyl transferase has been regarded as a prime target for anti caries therapy.39 Scientific studies and demonstrative analysis have emphasized on the aspect of sucrose metabolism by S. mutans which provides a better understanding of the oral clinical manifestation.40 Several enzymes associated with S. mutans enhance the sucrose utilization and these enzymes have been known to be connected with the extracellular region. Glucosyl transferase (GTF) is the major enzyme that favors the breakdown of sucrose and converts them in to adhesive glucans. This in turn contributes to significant amounts dental plaque formations which accumulate metabolic acids produced by bacterial colonies leading to demineralization of the enamel surface.41 GTF is a key component responsible for the formation of water soluble and insoluble glucose polymers through sucrose metabolism and also serves as a prime factor in triggering virulence. S. mutans is known to comprise of three kinds of GTFs that are responsible for the formation of water soluble and insoluble glucans. GTF B and C are accountable for the formation of water insoluble glucans and GTF D synthesizes water soluble glucans.
The unique feature of GTF B and C is the extent of similarities in their nucleic acid and amino acid sequences and are independent of glucan receptor and their enzyme activity is enhanced by the presence of dextran whereas GTF D is dependent on glucan acceptor. Demonstrative studies have also validated the prominence of components at the genetic level and its role in prompting virulence.42 The enzyme has a complicated network consisting of various domains including an independent functional domain which comprises of an amino terminal and carboxyl terminal. The amino terminal serves as the catalytic center which binds to sucrose where as the carboxyl terminal domain functions as glucan binding acceptor which plays a vital role in establishing the nature of glucan synthesized.43 The catalytic activity of the enzyme occurs in a sequential manner which includes sucrase activity and transferase activity. The sucrase activity involves the cleavage of sucrose in to fructose and enzyme bound glucosyl moiety. The reaction further proceeds as a consequence of subsequent transfer of glucosyl moiety due to transferase activity.44
Several demonstrative studies have indeed attempted to highlight the prominence of GTF enzyme and have deciphered many affiliated facets. The importance of gtf genes in cariogenesis was validated using some strains and one of those that have been studies in rat model is UA130 strain. It was revealed that S. mutans with a defective gene gtfB and gtfC genes resulted in reduced carious lesions. These genes are vital and are required for the synthesis of insoluble glucan which in turn contributes to smooth surface carious lesions. In addition to gtfB and gtfC, another vital gene associated with S. mutans is gtfD that codes for glucosyltransferase enzyme required for the synthesis of water soluble glucans. A mutant with a defective gtfD gene resulted in fewer smooth surface lesions when compared to UA130 strain of the same organism which had a fully functional counterpart of the same gene. Based on these finding it is quite obvious that these genes are very vital in terms of virulence which was substantiated through mutant studies on mutant strains.45
Streptococcus mutans which is regarded as a cariogenic bacterium often assumes sessile nature in response to sugars. It is believed that the biofilm formation exhibited by the microorganism is considered as one of the most thriving tactics employed for existence by the organism. It is in fact the environment which also decides the extent of survival and other associated requisites. The prominence of lactose in supporting the establishment of the microorganism by promoting biofilm formation has been substantiated by several studies. Studies have revealed the importance of lactose in enhancing the biofilm formation. Growth of S. mutans in different mediums with varying concentrations of lactose had no significant difference on the growth of the organism and this in turn has validated the role of lactose in biofilm formation. However, the extracellular polysaccharides as a consequence of lactose metabolism were different when compared to those produced as a consequence of sucrose breakdown. Hence, it is quite obvious that these sugars are vital and have their own significance in strengthening the organism in terms of growth, establishment and biofilm formation. Studies have also disclosed the role of lactose in up regulation of vital genes in S. mutans that may have a role in biofilm production and virulence.46 Therefore it could be understood that the process of establishment and virulence involves a cascade of reactions and a plethora of bio molecules which together contributes to the hostile nature of the organism.
The oral ecosystem favors the growth of a variety of microorganisms which ranges from mycoplasmas to fungi to bacteria and virus in certain extreme cases. These microbes continue to survive on the surfaces with the help of their biofilm forming abilities. This in turn contributes to the resident oral microbiome which resides in synchronization with the host. The oral microbiome also offers significant benefits from the context of overall health and well being. Marsh et al.,47 have validated the extent of proximity among the microorganism within the dental biofilms. Studies have also revealed the prominence of proximity among the microorganism towards a complicated level of interactions which could in turn lead to a positive or a negative outcome. The degree of microbial interaction is influenced by the conditions in the oral environment. Alterations could lead to altered microbial interactions which could in turn have a synergistic or an antagonistic impact on the host, thereby increasing the scope for oral manifestations.48,49 Intake of dietary sugar has been frequently associated with the formation of dental plaques because of their nature of rapid breakdown and formation of acids resulting in reduced pH within the biofilms.
Some studies have also validated the existence of symbiosis among the microorganism which enables them to manifest the normal conditions. Subsistence of these microorganisms within the biofilms has been made possible through the phenomena of symbiosis and this has been substantiated in some cultures. Experimental evidence of symbiosis in mixed cultures comprising of S. mutans and veillonella has been disclosed by several studies.50 Certain studies have also illustrated prominence of veillonella in inhibiting certain species in presence of an antagonist when added to mixed cultures. Veillonella added to a culture comprising of S. gordonii and S. mutans had a significant impact on the extent of sugar metabolism by S. gordonii and it also influenced the growth of S. mutans in the culture.51
The prominence of pH has been a vital facet from the context of the dental caries as experimental studies have disclosed an affirmative involvement in oral manifestations. Reduced pH will have a negative influence on those microfloras which positively contributes to enamel quality which in turn leads to the onset of dental problems. This condition also leads to reduced microbial diversity.52 According to Ebersole accretion of the microbial biomass within the vicinity of the gingival areas leads to an inflammatory response which in turn increases the flow of gingival cervicular fluid (GCF).53 However; the nutrient status also plays an important role in elevated GCF. GTF genes also have a vital role in strengthening the organism as these genes allows the breakdown of the sugar molecules resulting in acidic condition which could in turn lead to demineralization of the teeth. Certain studies also claim the functioning of these genes in collaboration and the inactivation of any of these genes would result in reduced smooth surface caries.54 According to Yamashita et al.,55 mutation in GTF genes have had an impact on the extent of glucan formation which is quite vital for the formation of dental caries and they also favor the adherence of the S. mutans on to the tooth surface which could later lead to dental caries. Therefore demonstrative studies have revealed a range of facets that allows the bacteria to cause oral manifestations.
Dental microbial communities are highly diverse and comprises of a range of microorganisms whose behavior depends on the oral ecosystem. Microbial communities affiliated with the dental biofilms are capable of exhibiting evolving features from the context of the microorganisms. In simple terms the microbial communities with the dental biofilms flaunts emergent aspects which is a consequence of a collaborative effort rather than individual organism. Hence is would not be appropriate to infer the characteristics of these microbial cities (biofilms) based on the characters of an individual organism.56 Studies have focused on S.mutans and its ability to survive within a community embedded inside the biofilms. The extent of survival rates of the organism relies on its ability to interact which could also be a factor for the onset of oral manifestations. The oral environment also plays a vital role in banking the organism. However, the tolerance level varies from one individual to the other and it’s also depends on the molecular entities in the organism that enables them to metabolize appropriate substrate leading to a convenient environment which could have a negative influence on the individual’s health as well as the oral microflora. Sucrose has been disclosed as one of the main substrates for the organism to survive but lactose has also been highlighted by certain studies. However further analysis is required to have a deep seated comprehension on facets like microbial interaction within the biofilms and the prominence of molecular entities.
None.
None.
Authors declare that there is no conflict of interest.

©2019 Ranganathan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.