Journal of
eISSN: 2373-437X


Research Article Volume 7 Issue 2
1School of Biological Sciences, Queen’s University Belfast, United Kingdom
2Food Microbiology Branch, Agri-Food and Biosciences Institute, United Kingdom
Correspondence: G Aboagye, Department of Nutrition and Dietetics, School of Allied Health Sciences, University of Health and Allied Sciences, Ho, Volta Region, Ghana, Tel 233(0)202993078
Received: February 06, 2019 | Published: March 14, 2019
Citation: Aboagye G, Rowe MT. Spectrophotometric determination of biofilm formation by Mycobacterium avium ssp. paratuberculosis in aqueous extract of schmutzdecke for clarifying untreated water in water treatment operations. J Microbiol Exp. 2019;7(2):72-76. DOI: 10.15406/jmen.2019.07.00245
Mycobacterium avium ssp. paratuberculosis (Map) causes Johne’s disease in ruminants, and implicated in the aetiology of human Crohn’s disease. The survival of Map in the environment and its ability to multiply inside a host has been reported, however, unknown by which mechanism. Biofilm formation by some species of mycobacteria is noted as a means of survival and host adaptation, with such knowledge lacking for Map. In this work, biofilm formation by 3 isolates of Map, 2 from the environment and 1 from a Crohn’s disease patient, was determined using spectrophotometric analysis. Since Map is fastidious and persists in water with or without nutrients, aqueous extract of schmutzdecke was employed to ascertain the impact of a complex microcosm of nutritional composition on biofilm formation by a fastidious slow growing Mycobacterium. Since cells must adhere on to a suitable surface in order to initiate biofilm formation, adherence assay was carried out firstly on 2 surfaces i.e. aluminium and stainless steel plates on which all the 3 Map isolates adhered, but greatly on the aluminium compared to the stainless steel. Secondly, biofilm formation by the isolates was determined on polyvinyl chloride (PVC) plates, and all were positive for biofilm formation. The extent of biofilm formation as influenced by distilled water (DW, control) alongside aqueous extracts of filtered and unfiltered schmutzdecke (FAES and UAES) was also determined statistically at P<0.05. The order of significance was 0.0004, 0.0307 and 0.0487 for DW, FAES and UAES respectively. This study showed that Map could form biofilm under conditions that its immediate environment provides, and could serve as a mechanism for its survival and thrive in the environment and host.
Keywords: adherence, biofilm formation, schmutzdecke, water treatment operations, Mycobacterium avium ssp. paratuberculosis
Mycobacteria possess a characteristic hydrophobic cell wall, and have the genetic competence to form biofilms.1‒4 They can attach and grow in biofilm on suitable substrata depending on growth requirements of the species involved, prevailing conditions in the environment during colonisation and properties of the substrata.5 For instance, some non-tuberculous opportunistic pathogens of mycobacterial species were recovered from biofilm associated with drinking water systems.6 Also, Schulze-Röbbecke & Fischeder7 and Schulze-Röbbecke et al.,8 recovered some species of both slow and rapidly growing mycobacteria in 45 of 50 biofilm samples taken from municipal or domestic water supplies in Germany and France. Pickup et al.,9 also found Map in biofilm on Nantgaredig Bridge spanning the River Tywi in Wales and on an abstraction site grating. These reports demonstrate the possibility that Map being a Mycobacterium, could also form biofilm since previous work has shown its occurrence and persistence in water treatment operations.10‒13 Furthermore, the presence of organic sediment, with which mycobacterial species are associated as part of biofilm microflora, is an important feature in their persistence in lakes and rivers.14,15 However, in the case of water treatment systems, the schmutzdecke which is a meshwork of biologically active matter atop a slow sand filter bed, may either promote or suppress the growth of mycobacteria. Even though to the authors knowledge, biofilm formation by Map in the water environment has not been reported, it does have a lipid cell wall16 which contains on the cell envelope glycopeptidolipids (GPLs) which convey hydrophobic properties.17 This makes Map potentially capable of attaching to a suitable surface as GPLs are directly or indirectly required for colonisation of some surfaces.18 In this study, biofilm formation by 2 environmental and 1 human isolates of Map was investigated under laboratory conditions, to establish their ability to form biofilm, in particular, in the water environment where schmutzdecke may provide proliferative support to environmentally persistent microorganisms for their consequential access to domestic water outlets.
Preparation of aqueous extract of schmutzdecke and map cultures
Distilled water was autoclaved at 121°C for 15 min and used as control and preparatory medium for the schmutzdecke. Aqueous extract of schmutzdecke [(AES; filtered (FAES) and unfiltered (UAES)] were prepared as follows: The FAES was prepared by filtering a portion of UAES through a 150 ml, 50 mm, 0.45 mm pore size filter unit (MF75™ Series, Nalgene, Fisher Scientific U.K. Ltd, Bishop Meadow Road, Loughborough, Leicestershire, LE11 0RG, UK). In the case of the UAES, 20 g schmutzdecke (obtained from a slow sand filter bed in a water treatment facility in Northern Ireland, UK) was smashed in 480 ml distilled water using Stomacher (Stomacher 400, Seward, England) for 4 min and particles allowed to settle (10 min) followed by centrifugation of the supernatant at 489 x g for 10 min to remove larger particles. Both the filtered and unfiltered AES portions were irradiated at a dose of 25 kGy and were stored at 4°C until required. Three Map cultures containing 106 CFU ml-1 were prepared from working cultures of Map ATCC 43015 (isolated from a Crohn’s disease patient) and L 85 and L 87 (isolated from raw water) with initial concentrations 108 CFU ml-1 (plate count), by centrifuging 1 ml of each working culture at 7558 x g for 20 min and reconstituting the pellets in 100 ml each of FAES and UAES and determining the CFU ml-1 on Middlebrook 7H10 plates.
Adhesion by map on aluminium and stainless steel coupons
One millilitre, each of three 6-week old working cultures of Map (Middlebrook 7H9 broth cultures of ATCC 43015, L 85 and L 87) were centrifuged (MSE Micro Centaus, Alpha Technologies, Larne, Co Antrim, Northern Ireland, UK) at 7558 xg for 20 min and the pellet resuspended in PBS-T20 to a final volume of 100 ml to prevent clumping of the cells. These were then incubated for 24 hours in 30mm Petri dishes in which were placed separately in a horizontal position 2 sterile coupons (autoclaved at 121°C for 15 min), each made of aluminium and stainless steel (dimensions: 2cm x 2cm) for 48 hours at 37˚C. Following the 2-day incubation period, the adhered cells were washed 3 times with distilled water before placing the coupons on a heating block at 100˚C for 2 min to heat-fix the cells. The coupons were then placed in Petri dishes (30mm) containing 0.03% (w/v) acridine orange dye, and incubated at room temperature (21˚C) for 20 min to allow penetration of the dye into the Map cell wall. The cells were washed again with distilled water to remove remaining acridine orange dye before air drying and subsequent inspection using x 1000 magnification of a fluorescent microscope (Humascope Flu LED with fluorescence filter; Germany), and one photographic record was made per run. The procedure was repeated at three independent times.
Biofilm formation by map
This procedure was followed for a period of 10 weeks. The media used were distilled water (DW, control), FAES and UAES. Using 100µl of the PBS-T20 reconstituted suspension of Map (ATCC 43015, L 85 and L 87, prepared as stated in Section 2.2), dedicated wells of three 96-well micro-titre plates made of PVC, flat bottom, Falcon 353912, Becton Dickinson and Company, Franklin Lakes, New Jersey, USA) were each added one isolate and then subjected to each of the media (DW, FAES and UAES) in separate wells to a final volume of 200µl. The dedicated wells of each plate were marked week 1 to 10, sealed with parafilm and incubated at 37°C for a period of 10 weeks. Cells in the wells per weekly designation were analysed for biofilms, and the volume of the remaining cultures maintained by replenishing them with 100µl of the respective media. This was to mimic a similar environment in water treatment operations where slow sand filter beds receive known volumes of water periodically to maintain the build-up of schmutzdecke. At the end of each week, remaining media was removed from each well, and 200µl of 1% (w/v) crystal violet (CV) was used to stain the cells for 30 min followed by three times of washing with the respective media. The optical density (OD) of cell-bound CV eluted into the wells after adding 200ml of 95% (v/v) ethanol to the wells for 1 hour, was measured by means of a Spectrophotometer (Sunrise Remote, Tecan, Austria, Gmbh, 5082, Grodig, Austria) using a wavelength of 595 nm. This experiment was repeated in triplicate runs and the average OD595nm values were calculated and plotted against time of incubation. The mean of the three runs for each isolate was calculated and used for statistical comparison by employing ANOVA, both between isolates and over time.
Adhesion by map on aluminium and stainless steel coupons
Figure 1 represents the adhered cells of Map (ATCC 43015) on aluminium whereas Figure 2 shows the same strain on stainless steel. Both coupons possessed different surface properties (rough for aluminium and smooth for stainless steel).
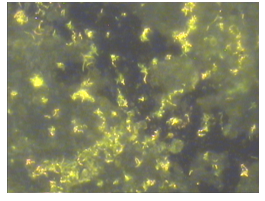
Figure 1 Adhesion of Map (ATCC 43015) to aluminium coupon analysed and photographed by fluorescent microscopy after incubation in PBS-T20 for 2 days at 37˚C, magnification x 1000.
Figure 2 Adhesion of Map (ATCC 43015) to stainless steel coupon analysed and photographed by fluorescent microscopy after incubation in PBS-T20 for 2 days at 37˚C, magnification x 1000.
Biofilm formation by map (ATCC 43015, L 85 and L 87) in DW (control)
An ANOVA showed a general significant difference (P < 0.05; 0.0004) in biofilm density amongst the 3 Map strains. The L 87 strain grew more cell mass compared to L 85 and ATCC 43015 strains. Thus, L 87 had a better response to growth in DW than the other two strains in the first 8 weeks of incubation before dropping in cell density in the following weeks (9 and 10). The L 85 and ATCC 43015 pattern however, followed an irregular trend with a generally low cell density between week 1 to 8 compared with L 87 (Figure 3).
Figure 3 Comparison of biofilm formation of Map (ATCC 43015, L 85 and L 878) in DW (control) showing the degree of variability in biofilm formation.
Biofilm formation by map (ATCC 43015, L 85 and L 87) in FAES
Figure 4 showed a significant difference (P<0.05; 0.0307) amongst the 3 strains in their response to biofilm density in FAES medium over the 10-week period. The response here also showed an increasing response of biofilm density by L 87 than L 85 and ATCC 43015. Unlike in the UAES medium, L 85 improved in biofilm density in the FAES medium than the ATCC 43015 which responded less.
Figure 4 Comparison of biofilm formation of Map (ATCC 43015, L 85 and L 878) in FAES medium showing the degree of variability in biofilm formation among the 3 Map isolates which was significant at P < 0.05.
Biofilm formation by map (ATCC 43015, L 85 and L 87) in UAES
In Figure 5, a general significant difference (P<0.05; 0.0487) in biofilm density amongst the 3 Map strains as influenced by UAES medium over the entire 10-week period was obtained. There was a greater increase in biofilm density by L 87 compared to ATCC 43015 and the least by L 85 at the end of the 10-week period. Looking at the graph, strains ATCC 43015 and L 85 appear to have a sigmoid response; one peaking at week 7, the other at week 5, and highest at week 10 by L 87. Thus both media (FAES and UAES), L 87 responded better followed by L 85 with ATCC 43015 responding less. It was noted that L 85 and L 87 grew denser biofilms in the aqueous extract of schmutzdecke than the ATCC 43015 at the maximum OD595nm.
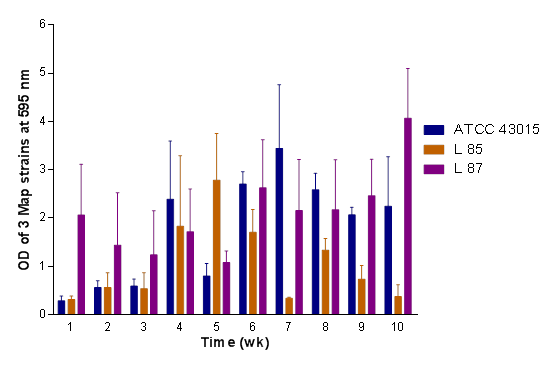
Figure 5 Comparison of biofilm formation of Map (ATCC 43015, L 85 and L 878) in UAES medium showing the degree of variability in biofilm formation among the 3 Map isolates at a significance level of P < 0.05.
Overall performance of DW, FAES and UAES on biofilm formation by the 3 map isolates
In Figure 6, the impact of DW, FAES and UAES on biofilm development of the 3 isolates were compared. The distilled water supported biofilm formation by the 3 isolates but was lower than FAES and UAES. The L 87 developed more biofilm over the 10-week period in both FAES and UAES than was the case for L 85 and the ATCC 43015. Weeks 1 to 3 seemed to have lagged in biofilm development with maximum optical density attained by L 87 whilst weeks 5 to 7 recorded denser biofilm for all the 3 isolates. The Crohn’s isolate (ATCC 43015) was comparable in density of growth to L 85 in FAES whilst L 85 recorded the least density at weeks 9 and 10. Thus, in general, the performance of the isolates in both media over the 10-week period was highest by L 87, and L 85 being slightly higher than ATCC 43015.
Adhesion of map cells to aluminium and stainless steel coupons
Aluminium and stainless steel materials used in water pipes and water treatment works were chosen as substrata for this laboratory-based biofilm studies. The ATCC 43015 strain of Map was also chosen for this work as the reference strain partly because it was obtained from a human source and has also been cultivated in the laboratory over a period, hence considered as having a wider adaptation to different environments. There is no doubt that mycobacteria form biofilm; examples are M. kansasii,19 M. flavescens7 and M. ulcerans.20 However, to the author’s knowledge there is no published information on Map forming a biofilm under environmental conditions. Mycobacterium kansasii and M. marinum are slow growing and pathogenic like Map while M. flavescens is fast growing and non-pathogenic. Also, there is circumstantial evidence that a close relative of Map i.e. M. avium ssp. avium can form biofilm in that it has been reported to persist in actively flowing streams3 presumably as a result of forming biofilm on the rocks present. It is clear that the isolates adhered to both surfaces after 2 days of incubation. In particular, there was a greater density of cells on the aluminium coupon than the stainless steel coupon.
Biofilm formation by map isolates
Following research reports that mycobacteria could form biofilm, and success of the adhesion test with the Map isolates, it seemed prudent to evaluate biofilm formation by Map, and the extent to which this was a common trait amongst its isolates as these were not reported in literature. Whilst 37˚C provided optimum growth temperature for Map, the question as to whether the three isolates would behave or grow optimally when environmental components of water treatment works were also present needed to be answered. The environmental components chosen were FAES and UAES. The two Map isolates (L 85 and L 87) showed better growth in both the FAES and UAES over the ATCC 43015 strain frequently at specific weeks over the entire period, perhaps to be expected since they are environmental isolates and may have little or no nutrients in the environment compared to the schmutzdecke which builds up over time on top of a slow sand filter. However, the FAES and UAES media suspensions are rich in both humic and fulvic acids which partly serve as metabolic nutrients for environmental M. avium complex in the coastal areas.21,22 Since the L 85 and L 87 strains were obtained from the water environment, they might have derived and better assimilated these metabolic nutrients than the ATCC 43015 strain, agreeing to a possible variation in biofilm formation by Map isolates,23 with the ATCC 43015 peaking growth around week 7 in all the three media. It can be seen that all the three strains of Map showed optimum growth after 5 weeks, however, the decline in growth following this duration of time, may be attributed to production of metabolites that were inhibitory to growth. However, L 87 generally had more biofilm growth in both FAES and UAES in weeks 9-10 compared to the L 85 which also grew a denser biofilm than the ATCC 43015 strain. It is worth commenting that there was generally no difference in growth response between FAES and UAES. Also, the make-up of the PVC plate employed for this work may have contributed to the biofilm formation by the 3 strains, by providing a suitable substratum24 since more substantive mycobacterial biofilm have been shown to occur on plastics and rubber compared to other substrata.8
The water environment may provide a conducive habitat for Map to reside and form biofilm compounded by factors such as availability and variation in nutrient composition and substratum. Whilst Map has been shown to form biofilm greatly on aluminium, this material could be limited where applicable in water treatment operations. Also, where slow sand filters are used, the schmutzdecke should be monitored and scraped periodically to prevent its proliferative support for waterborne pathogens and those that generally persist in the water environment.
This work was fully supported by the following scholarships from Queen's University of Belfast, Northern Ireland, United Kingdom: Gibson, Harold Barbour and Mac Geough Bond.
All the necessary considerations on ethics were fulfilled for this work
The authors wish to express their gratitude to Queen’s University Belfast, for funding this research, and Agri-Food and Biosciences Institute for allowing access to their laboratories.
No conflicts of interest were associated with this work.

©2019 Aboagye, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.