Journal of
eISSN: 2373-437X


Research Article Volume 7 Issue 1
Aligarh Medical University India
Correspondence: Nusrat Perween, Aligarh Medical University, Doharra, Near SIG Hospital, Aligarh, UP, India, Tel 8791325355
Received: January 09, 2019 | Published: February 7, 2019
Citation: Perween N, Khan HM, Fatima N. Silver nanoparticles: an upcoming therapeutic agent for the resistant Candida infections. J Microbiol Exp. 2019;7(1):49-54. DOI: 10.15406/jmen.2019.07.00240
Objective: To elucidate the antifungal activity of silver nanoparticles on the clinical isolates of Candida albicans and to compare the performance of silver nanoparticles with the conventional antifungals.
Materials and methods:
Sample size: The present study was carried out in the Department of Microbiology, JNMCH, AMU.6000 patients were included in the study. Samples were collected according to their clinical presentation.
Identification of Candida species: It was done as per the standard protocol.
Testing of conventional antifungals susceptibility by (i)Disc diffusion (ii)Broth microdilution method
Evaluation of antifungal activity of Silver nanoparticles and Comparision of its performance by (i)Well diffusion (ii)Broth microdilution (iii)Turbidometry method
Results: In 6000 samples, 103 Candida albicans were isolated.Most of these isolates were resistant to either Azoles, Nystatin or Amphoteracin B and 10 of them were resistant to all. But they formed zones of inhibition with varying concentrations of silver nanoparticles (AgNP) in well diffusion method, confirming antifungal activity of AgNPs. On comparing the MIC of AgNP and other conventional antifungals, revealed very low MIC of AgNP (0.125-0.5µg/ml) as compared to fluconazole (1-64µg/ml) and caspofungin (0.062-1µg/ml).
Conclusion: Silver nanoparticles have more potent antifungal activity in vitro on the resistant Candida albicans isolates.
Keywords: Candida albicans, silver nanoparticles, antifungal activity, well diffusion, broth microdilution, Turbidometry
In comparison to bacterial pathogens, fungi were less frequently the cause of infectious diseases in humans earlier. However, with the increased number of immunosuppressed patients, fungal infections have gained enormous medical importance. And, today Candida spp. have become common nosocomial pathogens and even serious systemic Candida infections may frequently lead to death and represents a serious public health challenge with increasing medical and economic importance due to the high mortality rates and increased costs of care and duration of hospitalization.1,2 Intensive use of antifungal drugs has led to an incessant increase in the number of resistant fungal strains retaining viability due to their resistance mechanisms.3 Initially sensitive Candida spp. were shown to acquire resistance to various antifungal agents, after gradual exposure to increasing concentrations of these agents. This development of resistance was found to be due to mutation.4 Pfaller et al.,5 reported that exposure of C.glabrata to subtherapeutic concentration of fluconazole may result in resistance.
In recent years, a rapid increase in microbes that are resistant to conventional antibiotics has been observed worldwide. In India, there are very few studies regarding antifungal susceptibility pattern. So, here in this study we are going to study the resistance pattern in Candida isolates. There have been a few reports of strains of C. albicans showing resistance to amphotericin B and azoles. But irrespective of their resistance to azoles or amphotericin B,6 results of a global surveillance which dealt with trends in the susceptibility of Candida spp. to Caspofungin found no evidence for a shift in the Caspofungin MIC distribution.7 A recent study by Lee et al.,8 in BALB mice has found that the efficacy of caspofungin against C. albicans was reduced in vivo due to either elevation of chitin levels in the cell wall or acquisition of FKS1 point mutations. Resistance to echinocandin therapy has been associated with amino acid substitutions in FKS1 (Candida albicans, Candida tropicalis, Candida krusei, Candida glabrata) and FKS2 (C. glabrata).9,10 In such situation, we should now search for better alternative antifungal agent.
The use of nanoparticles is gaining impetus in the present century. Among them, the metallic nanoparticles are most promising and which is of interest to researchers due to the growing microbial resistance against metal ions, antibiotics and the development of resistant strains.11 The silver ion exhibits broad spectrum biocidal activity toward many different bacteria and viruses. Silver is known to cause pits in bacterial cell walls, leading to increased permeability and cell death and is believed to be the active component in silver-based antimicrobials.12 Though, the biocidal effect and mode of action of silver ion are not known, the antifungal effect of silver NPs has received only marginal attention and just a few studies on this topic have been published.13-15 So, this study is aimed on the determination of the antifungal effects of the silver NPs against selected pathogenic yeasts like Candida and its comparison with other antifungals.
Study group
The present study was carried out in the Department of Microbiology J. N. Medical College, AMU. The cases selected for the study include all clinically important immunocompromised patients susceptible to Candida infection, irrespective of age and sex. Various clinical specimens including skin swab, nails, oral swab, cervical swab, urine, sputum, BAL, Endotracheal aspirate CSF, pus and blood culture were collected. The specimens were obtained using standard microbiological techniques for fungal organisms.
Direct microscopy
Specimens like endotracheal aspirate, urine, oral swab etc., were subjected to direct microscopy by making a KOH mount and/or a Gram stained smear.
Fungal culture
The culture was done on two sabouraud dextrose agar (SDA) slants containing chloramphenicol (0.05mg/ml) by rolling over the surface and subsequently in BHI broth also. One tube was incubated at 25oC and the remaining tube and BHI broth were incubated at 37oC.The isolates were identified in accordance to (i) Colony characteristics, (ii) Germ-tube test (GTT test), (iii) growth at 42oC, (iv) morphology on CMA, (v) Sugar fermentation tests and (vii) Sugar assimilation tests.16-18
Antifungal susceptibility testing of conventional antifungals
Antifungal susceptibility testing was performed by the disc diffusion and broth microdilution methods.
Disc diffusion method: Antifungal susceptibility by the disc diffusion method was performed according to CLSI document M44-A2. The antifungals tested were Amphotericin B (100µg), Nystatin (100µg), Ketoconazole (10µg), Clotrimazole (10µg), Fluconazole (25µg), Itraconazole (10µg) and Caspofungin (5µg, prepared in house).Test strain was considered Sensitive–when the zone diameter was ≥80% of the zone diameter of the control strain. Intermediate-when the zone diameter was <80% but there is visible zone of inhibition. Resistant–when there was no zone of inhibition.
Broth micro dilution method: Broth micro dilution method was adopted in this study as per CLSI (2008) guidelines based on document no. M-27A3.20 The antifungal tested were Fluconazole and Caspofungin with the drug concentrations mentioned in Table 2 and Table 3. MICs were calculated as the lowest concentrations at which there was 80% inhibition of growth compared with that in a drug free control. Control broths were used without drugs. ATCC 24433 Candida albicans is included as the control organism each time with each drugs.
Evaluation of antifungal activity of nanoparticles
Nanoparticles: Commercially prepared nanoparticles were obtained with known size and other specifications. Silver nanoparticles used was purchased from Sigma Aldrich, (product no; 576832) which had a particle size of less than 100nm.
Preparation of nanoparticle solution: Silver nanoparticles were brought in dispersion form with known concentrations from which different concentrations were made by dissolving them in distilled water. Nanofluids prepared were autoclaved at 121ºC for 20 min and then cooled down to the room temperature.
Well diffusion method: The agar well diffusion was done using the method by Valodkar M et al.,21 with some modifications in this study. The saboraud dextrose agar for fungi seeded with the test organisms were punched with a sterile cork borer (0.5cm diameter) to make open wells. AgNPs were added into the open wells at different concentrations (0.062, 0.125, 0,250, 0.5, 1µg/ml). The plates were incubated at 37°C at 25°C for max. 6 days for C. albicans. The same test is performed with ATCC 24433 Candida albicans as the control with the same drug concentrations. The zones of inhibition were measured in mm and recorded. The lowest concentration of AgNPs that inhibited the growth of the test organisms was recorded as the minimum inhibitory concentration (MIC).
Broth micro dilution method: Broth micro dilution method was performed using the methodology similar to that used for antifungal MIC studies. And standardisation and dilutions of silver nanoparticle was done based on study by Sultan et al.,22 with slight modifications. The MIC was determined in LB broth using serial two-fold dilution of Silver nanoparticles in concentrations ranging from 0.062-1µg/ml .Testing was performed in 96-well round-bottom microtiter plate. Cell suspensions were prepared in BHI medium and were adjusted to give a final inoculum concentration of about 0.5×103 to 2.5×103 cells/ml. Finally, 10μL of the fungal suspension was added to each well. The plate was incubated at 35°C and was read after 48 h (Candida spp). Control broths were used without nanoparticles. ATCC 24433 Candida albicans is included as the control organism each time with each drugs.The MIC was determined as the lowest concentration of silver nanoAg giving no visible growth or causing almost complete inhibition of growth.
Turbidity measurement of fungal growth by using spectrophotometer: This test was performed according to the study of Sultan et al.,22 with slight modifications. Before the test was performed, the Candida albicans isolates were freshly subcultured just 1 day before the test was to be performed. Isolates were inoculated in 100 ml of Luria– Bertani (LB; HiMedia) culture medium. Growth was allowed until the optical density reached 0.1 at 620nm (OD=0.1, which corresponds to 108 CFU/ml of the medium). Subsequently, 2×108 CFU/ml from above was added to 100ml of liquid LB media supplemented with concentrations ranging from 0.062-1µg/ml of AgNPs. All the flasks were put on rotatory shaker (150 rpm) and incubated at 37°C. Control broths were used without nanoparticles. Fungal growth was determined by measuring the optical density after every 4 h (up to 16 h) at time intervals of 0, 4, 8, 12, 16, 20 and 24 hrs at 620 nm using a spectrophotometer (VSP66, LOBA Life, India). Growth curves were then plotted.
Out of a total of 6000 patients, Candida was isolated in 103 (1.7%).Among the total Candida isolates, 68(66.02%) were C. albicans and remaining 35(33.98%) were non- albicans Candida. Resistance was observed in 26.5% isolates to Fluconazole, 27.9% isolates to Ketoconazole and Clotrimazole, 23.5% isolates to Itraconazole, 11.8% isolates to Amphotericin B, 1.5% isolates to Nystatin and 0 % isolates to Caspofungin via disc diffusion method (Table 1).
|
Antifungal agent |
Sensitive |
Resistant |
|
Clotrimazole |
49(72.1) |
19(27.9) |
|
Fluconazole |
50(73.5) |
18(26.5) |
|
Amphotericin B |
60(88.2) |
8(11.8) |
|
Nystatin |
67(98.5) |
1(1.5) |
|
Ketoconazole |
49 (72.1) |
19(27.9) |
|
Itraconazole |
52(76.5) |
16(23.5) |
|
Caspofungin |
68(100) |
0(0.0) |
Table 1 Susceptibility pattern of Candida isolates to various antifungal agents
Figures in parenthesis indicate percentage
By Broth microdilution, 22.2 % isolates of Candida albicans were resistant to fluconazole and 5.6% isolate were dose dependent sensitive and the MIC range was between 1-64µg/ml (Table 2).|
|
|
MIC of fluconazole (µg/ ml) |
Total |
||||||||
|
0.125 |
0.25 |
0.5 |
1 |
2 |
4 |
8 |
16 |
32 |
≥64 |
||
|
C. albicans |
- |
- |
12(66.7) |
- |
- |
- |
- |
1(5.6) |
4(22.2) |
18(100) |
|
Table 2 MIC values for fluconazole by broth dilution method
Figures in parenthesis indicate percentage
But no isolates of Candida albicans were resistant to Caspofungin and its MIC range was between 0.062-1µg/ml. Among 103 C. albicans isolates, 10(9.7%) were resistant to all Azoles, Amphoteracin B and Nystatin.All(9.7%) of those isolates that were resistant to all conventional antifungals showed zone of inhibition with silver nanoparticle(AgNP) in Well diffusion method. Thus, we can say that antifungal activity is present in silver nanoparticle. It was also observed in Well diffusion method that 20% isolates of C. albicans had MIC value of 0.5µg/ ml for AgNP, 50% had a MIC value of 0.25µg/ ml, 30% isolates had MIC value of 0.125µg/ml. Thus, the MIC range of these isolates varies between 0.125 to 0.5µg/ml.
With Broth microdilution, it was observed that AgNP had MIC value of 0.5µg/ml for 10% isolates of C. albicans, for 70% of them, MIC value was 0.25µg/ml and for 20% isolates it was 0.125µg/ml. Thus, the MIC range of AgNP for these isolates varies between 0.125 to 0.5µg/ml (Table 3).
|
|
MIC of AgNP(µg/ ml) |
Total |
||||
|
0.062 |
0.125 |
0.25 |
0.5 |
≥1 |
||
|
C. albicans |
- |
2(20) |
7(70) |
1(10) |
- |
10(100) |
Table 3 MIC values for Silver nanoparticle by broth dilution method (n=10)
Figures in parenthesis indicate percentage
The susceptibility testing of AgNP and other conventional antifungal agents by broth dilution method revealed very low MIC of AgNP (0.125-0.5µg/ml) as compared to fluconazole (1-64µg/ml) and caspofungin (0.062-1µg/ml). Thus, AgNP proved to be more potent than other antifungals in vitro (Table 4).
|
Antifungal agent |
MIC Range in the same isolates of study group (µg/ml) |
|
Fluconazole |
1-64 |
|
Silver nano-particle |
0.125-0.5 |
|
Caspofungin |
0.25–1 |
Table 4 Comparison of sensitivity of Silver nanoparticle with fluconazole
On studying the effect of various concentrations (0.062-1) of AgNPs on the growth curve of Candida albicans spectrophotometrically, the AgNP caused a significant growth delay (Figure 1 and Figure 2).
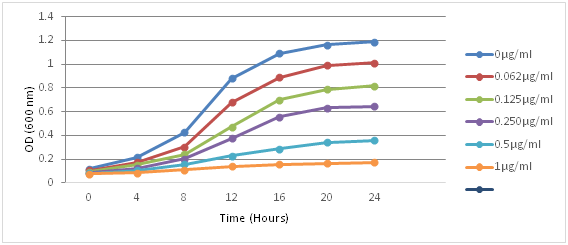
Figure 1 Represents growth curve of Candida albicans ATCC 24433 strain, in LT Broth inoculated with 0.5x103 to 2.5x103 cells/ml of fungus in the presence of silver nanoparticles. There was very little antifungal activity at concentrations of 0.062 and 0.125µg/ml but profound effect was seen at concentration of 0.5 and 1µg/ml. Silver nanoparticles with highest concentrations showed maximum delay in growth curve in 24 hrs.
Overall the rate of Candida isolation from various specimens in our study group was 1.7%. C.albicans formed the largest group (66.02%) of Candida species isolated in this study. This observation is consistent with that of various works which reports nearly identical reports. Pfaller23 had reported 50 to 70% Candida albicans isolation, Wingard24 54%, Roilides et al.,25 65%, Pfaller et al.,26 66% of isolation in their respective studies. Indian studies which also reported similar findings were Narain et al.,27 (53.3%), Kaur et al.,28 (50%). However, Kotwal et al.,29 noted a much higher prevalence of C.albicans (78.1%).
However certain other studies showed non albicans Candida as the most frequently isolated species. This species variation may be due to the differences in empiric or prophylactic practices. In our study, resistance was observed in 22.2% isolates to fluconazole, 27.9% isolates to ketoconazole and Clotrimazole, 23.5% isolates to Itraconazole and 11.8% isolates to amphotericin B. 5% isolates to Nystatin and none to caspofungin. These findings are in agreement with the study conducted by Kotwal et al.,29 and Narang et al.,30 who found a higher rate of fluconazole resistance (24% and 26% respectively). And in contrast to the study by Xess et al.,31 who reported 11.7% resistance to fluconazole and Belet et al.,32 (8.5%) and Rizvi et al.,33 (10.3%).
In this study, all the isolates (100%) were susceptible to Caspofungin. This finding is similar to the study of Pfaller et al.,34 who determined the in vitro activity of caspofungin against 351 fluconazole resistant Candida isolates and reported that 99% were susceptible to caspofungin. But, A recent study by Lee et al.,5 in BALB mice has also found that the efficacy of caspofungin against C. albicans was reduced in vivo due to either elevation of chitin levels in the cell wall or acquisition of FKS1 point mutation. Most of the antifungal agents are limited in clinical applications because of their complications. For example Amphotericin B has bad effect on kidneys and leads to renal failure after using these drugs. The Azoles family like Fluconazole, cause liver toxicity and inhibits testosterone synthesis. So, new drugs with less complication are need of the hour. Also, an alternative way to overcome the drug resistance of various microorganisms is needed desperately.
These days’ works on nanoparticles are being carried out using it as a therapeutic agent. And, among different nanoparticles, Nanosilver is the most mentioned nanomaterial and is evolving as the most effective nanoparticle with variety of applications.35 Silver nanoparticles possess increased antifungal activity against Candida species.36-38 In this study, antifungal activity of silver nanoparticle was studied using well diffusion method on those Candida albicans isolates which were resistant to other conventional antifungals. And it was found that all those isolates formed zones of inhibition with various concentrations of silver nanoparticles (AgNP). Thus, it proved the antifungal activity of AgNP on Candida albicans isolates in vitro. Similarly, Roe et al.,39 have tested the anti-fungal activity of plastic catheters coated with Ag-NPs and results showed that the growth inhibition was almost complete for C. albicans.
Results of the susceptibility testing by broth dilution for silver nanoparticle showed MIC range of 0.125–0.5µg/ml against Candida albicans isolates. Similar results were seen in 2009 by Panacek et al.,40 who investigated the antifungal activity of Ag-NPs prepared by the modified Tollens process. Results revealed the minimum inhibition against C. albicans growth at 0.21-0.42mg/ml using naked Ag-NPs. Monteiro et al.,41 also reported MICs of Ag-NPs from 0.4 to 3.3 μg/ml against C. albicans adhered cells and biofilm. In our study we also compared the antifungal activity of AgNP with other antifungal agents and we found AgNP to be more potent agent in vitro than other antifungals. Recently, Nasrollahi et al.,42 also reported the antifungal activity of AgNPs against C. albicans. AgNPs had been used as a comparable antifungal drug against others like Amphotericin B and Fluconazole. They found MIC90 of Amphotericin B, Fluconazole and AgNPs on Candida albicans were 4mg/ml, 16mg/ml, 2mg/ml respectively. Also, Hassan et al.,43 found that the MIC100 of the antifungals like Grisofulvin, Itraconazole and Ag-NPs were 7±1.2µg/ml, 13±3.0µg/ml and 4±2.0µg/ml, respectively against C.albicans. Thus, both of them also found AgNP to be more potent agent than other antifungals.
Silver nanoparticle is a newer therapeutic option for treating resistant cases of candidiasis. It has considerable antifungal activity in vitro, but deserves further investigation in vivo, proper standardization & stabilisation and also studies on its toxicology to get its efficacious drug concentration for clinical applications.
None.
Authors declare that there are no conflicts of interest.

©2019 Perween, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.