Journal of
eISSN: 2373-437X


Research Article Volume 5 Issue 3
National Dairy Research Institute, Deemed University, India
Correspondence: Raghu HV, Dairy Microbiology Division and National Referral centre, ICAR-National Dairy Research Institute, Deemed University, Karnal-132001, India, Tel 91-184-2259534, +91-9466963599
Received: May 09, 2017 | Published: July 19, 2017
Citation: Raghu HV, Kumar N, Mayuri, Arya K, Sharma PK (2017) Screening of Lectins for Specific Detection of Listeria Monocytogenes. J Microbiol Exp 5(3): 00150. DOI: 10.15406/jmen.2017.05.00150
In the present study, a specific binding activity between lectin and Listeria monocytogenes was studied by agglutination assay. The ten lectins were screened for their agglutination activity (HA) against L. monocytogenes ATCC 15313, 19111, 19115, 19118, 13932 & BAA 751 along with lab isolates of RM –17 and RM 26. Wherein 84.4±1.76% binding was observed with wheat germ agglutinin (WGA) this was followed by 70% with Helix pometia agglutinin(HPA), 51.25% with Arachis hypogea lectin (AHL), 39.375% with Ulex europeas lectin (UEL), 30.625% with Lens culinaris lectin (LCL), and 18.75% with Sambucus nigra agglutinin (SNA), 26.25% with Phytolaccka Americana(PAL), 31.25% Maackia amurensis lectin (MAL) and 16.25% concavalin A. But, <10% HA was observed with Pisium Sativum (PSA). Additionally, these lectins were also screened for binding activity with Gram positive and Gram negative bacteria other than L. monocytogenes, but, no agglutination was observed except Salmonella (10–20%), Klebsiella (10–20%), and Citrobacter (45%). The HA of WGA with L. monocytogenes was observed under scanning electron microscopy having cell aggregation with gapped and patchier cells, but, in some cases cell rupture was also seen. It was clear from the sugar inhibition assay that the HA of WGA with L. monocytogenes was reliably inhibited in presence of N–acetylglucosamine (NAGA) at a rate of 0, 35, 50, 80 and 100%, respectively, at 0, 0.1, 0.25, 0.5, 0.75 and 1.0 M concentration, respectively. Whereas in the case of glucose, the % inhibition was less compared to WGA followed by galactose, in the case of mannose, a least inhibition of 25% was observed at 1 M concentration. The complete inhibition of agglutination in the presence of NAGA indicates that the binding of L. monocytogenes cells to WGA lectin was NAGA specific. These results will help in the use of WGA in the development of rapid test for detection of L. monocytogenes in milk.
Keywords: lectin, listeria monocytogenes, agglutination
WGA: Wheat Germ Agglutinin; PSA: Pisium Sativum; HPA: Helix Pometia Agglutinin; SNA: Sambucus Nigra Agglutinin; PAL: Phytolaccka Americana; LCL: Lens culinaris Lectin; UEL: Ulex Europeas Lectin; MAL: Maackia Amurensis Lectin; FSSAI: Food Safety Standard Authority of India; ATCC: American Type Culture Collection; EMCCD: Electron Multiplying Charged Coupled Device
Listeria monocytogenes is the causative agent of listeriosis, a disease that can be serious and is often fatal in high risk groups such as pregnant women, neonates, and immunocompromised adults with a rate which may reach 75%.1 Different food types including raw vegetables, raw milk, soft cheeses, fish, poultry, processed chicken, and beef have been found to be contaminated with L. monocytogenes.2 In particular, the ability of the organism to grow at refrigeration temperatures.3 and on dry surfaces.4 and its ability to tolerate acidic conditions make it well adapted to food environments which normally restrict bacterial growth. The primary sources of L. monocytogenes in milk and dairy products include the feed, bedding, vegetation, soil, animal faeces, contaminated water, diseased and unclean udders and teats, human hands and handling equipment.5 Both facts, i.e. occurrence of L. monocytogenes in food.6 and the risk of contracting listeriosis have been very well established. The linkage of dairy products to both invasive and noninvasive listeriosis outbreaks is well known.7 Various outbreaks have been associated with raw milk, pasteurized milk.8 soft cheeses.9 Crave Brothers Farmstead cheeses.10 hard cheese.11 semisoft cheese.12 and ice cream.13 (CDC, 2015). The food safety regulations of some countries (e.g. USA) require zero tolerance of L. monocytogenes in ready–to–eat (RTE) foods.14 For others, therefore, L. monocytogenes has also become an issue of global concern because of its increased presence in milk and other food products.15 Recently, Food Safety standard Authority of India (FSSAI) has established a microbiological criteria for dairy products that L. monocytogenes should be absent in per 25g of every dairy product in India.
Conventional methods for the detection of food pathogens, although typically sensitive, often require multiple time–consuming steps such as extraction, isolation, enrichment, counting, etc., prior to measurement, resulting in testing times which can be in days.16 Rapid detection of foodborne pathogens is a key step in the control of food related diseases. There is an urgent necessity to develop rapid and sensitive detection methods. The advent of new technologies, namely biosensors, has brought in new and promising approaches comparable to or better than the conventional analytical systems in terms of performance (e.g. reliability, sensitivity, selectivity, specificity and robustness), that can identify the contaminants much faster, more efficiently and can give effective real–time monitoring.
A range of molecules with bio–recognition powers are available naturally such as antibodies, enzymes, and nucleic acids, which are used in the development of rapid techniques for detection. Lectins are carbohydrate–binding proteins other than enzymes or antibodies that exist in most living organisms, ranging from viruses and bacteria to plants and animals.17 In particular, their role in cell recognition, as well as their employment as invaluable tools for the study of complex carbohydrates in solution and on cell surfaces, is contributing markedly to advances in glycobiology. Lectins interact with carbohydrates, non–covalently in a manner that is usually reversible and highly specific.18 Specially, plant–isolated lectins that are well characterized and used as new bioreceptors play a pivotal role in many biosensing devices due to their exquisite specificity for their cognate carbohydrates on the bacterial cell surfaces.19 Furthermore, the molecular size of lectins is much smaller than antibodies; thus, they allow higher densities of carbohydrate–sensing elements leading to higher sensitivity and lower nonspecific adsorption.20 Therefore, in our present investigation, we screened different plant lectins for the rapid detection L. monocytogenes in dairy products.
Procurement and maintenance of culture
Different strains of Listeria monocytogenes 15313 (serotype 4b), 13932 (serotype 4b), 19111 (serotype 1/2a), 19115 (serotype 4b), 19118 (4e) and BAA 751.American type culture collection (ATCC), Manassas, Virginia), L. ivanovii 19119 (ATCC), Listeria innocua 33090 (ATCC), Enterococcus faecalis 14506 (ATCC), Staphylococcus aureus (ATCC), Bacillus cereus 14569 (ATCC), Lactobacillus GG.National collection of Dairy cultures (NCDC), E. coli 25992 (ATCC), Salmonella choleraesuis 10708 (ATCC), Enterobacter aerogenes 13048 (ATCC), Klebsiella pneumonia 138 (NCDC), Citrobacter freudenii ATCC 43864, Yersinia enterocolitica ATCC 23715 and Serratia marcescens 13880 (ATCC) used in this study were procured from their respective mentioned sources and checked for purity. Cultures were activated by incubating at 37oC for overnight followed by streaking on respectively; selective agars reported in Table 1, which were procured from Himedia Chemicals, Mumbai. Incubations were done overnight at 37OC in a shaker incubator (New Brunswick™ Innova® 42/42R, Eppendorf, Germany). A single pure colony from a respective selective medium after microscopic examination was done to check the purity of the culture was picked up and maintained on its respective maintenance media by routine sub–culturing after every 15 days. Pure colonies from a selective medium of each test organism was transferred to 5.0 ml of respective maintenance broth (Table 1) and incubated for 24 hrs at 37°C. The culture broths were then centrifuged at 8994×g (5810R, Eppendorf, Germany) for 5 min at 10°C. The cell pellet was washed once with 10mM phosphate buffer (PB), pH 6.8 in 2.0mL eppendorf tubes to eliminate broth components. Final suspension for each test organism was prepared in 10mM PB, pH 6.8 and the concentration of each organism was set to an OD of 1.0 using Tecan M pro 200 multimode plate reader (Bioscreen Instruments, Chennai, India) for further agglutination assays.21,22
|
Cultures |
Strains |
Procured from |
Maintenance Broth |
Selective Media |
Maintenance Media |
|
Listeria |
L. monocytogenes 15313, 13932, 19111, |
ATCC |
Brain Heart Infusion |
Polymyxin-Acriflavin-Lithium |
Brain Heart Infusion agar |
|
Enterococci |
E. faecalis 14506 |
ATCC |
Citrate azide agar |
BHI slants |
|
|
Lactobacilli |
Lactobacillus GG 347 |
NCDC |
deMann Rogosa Sharpe (MRS) |
MRS agar |
MRS agar slants |
|
Bacillus |
Bacillus cereus 11778 |
ATCC |
Nutrient broth |
Bacillus cereus Agar |
Nutrient agar (NA) slants |
|
Staphylococcus |
S. aureus 9144 |
ATCC |
Brain Heart Infusion |
Baird Parker Agar |
BHI agar slants |
|
Escherichia |
E. coli 25922 |
ATCC |
Nutrient broth |
Violet red bile agar (VRBA) |
NA slants |
|
Salmonella |
S. choleraesuis 10708 |
ATCC |
Xylose lysine deoxycholate agar |
NA slants |
|
|
Enterobacter |
E. aerogenes 13048 |
ATCC |
VRBA |
NA slants |
|
|
Klebsiella |
K. pneumoniae 138 |
NCDC |
VRBA |
NA slants |
|
|
Citrobacter |
Citrobacter freundii |
ATCC |
VRBA |
NA slants |
|
|
Yersinia |
Y. enterocolitica 23715 |
ATCC |
VRBA |
NA slants |
|
|
Serretia |
S. marcescens 14756 |
ATCC |
VRBA |
NA slants |
Table 1 Selective and maintenance medium used for L. monocytogenes and other contaminants [need to spell out the acronyms used for maintenance broth, selective medium, and maintenance media]
Screening of lectin for the specific binding with L. monocytogenes
Ten lyophilized lectins (1mg/mL) such as Canavalia ensiformis (Con A), wheat germ agglutinin (WGA), Helix pomatia agglutinin (HPA), Pisium sativum (PSL), Arachis hypogaea (PNA), Ulex europaeus (UEL), Lens culinaris (LCL), Sambucus nigra (SNL), Maackia amurensis (MAL) and Phytolaccka Americana (PAL) were procured from Sigma Aldrich Chemicals Pvt Ltd., (Bengaluru, India) and dissolved individually in PB (10mM, pH 6.8) followed by dilution to an optimum concentration of 200μg/ mL. About 50μL of each lectin mixed with 50μL of L. monocytogenes cells at a concentration of 107 CFU per ml.(15313 (4b), 13932 (4b), 19111 (1/2a), 19115 (4b), 19118 (4e) & BAA 751, RM–17 and RM–26) in suspension were added together in U bottom microliter plate procured from Cole–Parmer (Mumbai, India). For a control, L. monocytogenes suspension was mixed with 50µl of PB in separate wells of U Bottom microliter plate. All the experiment related to screening of lectin was done in triplicate (n–=3). The microtiter plate was incubated at 37oC for 10min in a shaker incubator (New Brunswick™ Innova® 42/42R, Eppendorf, Germany). After incubation, the microtitre plate was observed for agglutination visually.21 with the appearance of ground glass in transmitted light was taken as a negative reaction. Further, agglutinated cells were stained with crystal violet dye (Himedia, Mumbai, India) and observed under iXon Electron Multiplying charged coupled device (EMCCD) using light microscopy (ANDOR, Bengaluru). Images of agglutination were captured using a camera Sony Lightshot (Sony New Delhi, India). The microscopic observation taken during the agglutination assay was interpreted as per Naughton et al.22 who described 80–90% agglutination as +4, 60–70% agglutination as +3, 40–50% as +2, 10–20% as +1 and < 10% as negative.
Screening of lectin with gram positive and gram negative bacteria
Further, all ten lectins were screened individually with different Gram positive bacteria (GPB) other than L. monocytogenes (Listeria ivanovii, Listeria innocua, Bacillus cereus, Staphylococcus aureus, Lactococcus lactis, Lactobacillus fermentum, Leuconostoc mesenteroides) and Gram negative bacteria (GNB).Escherichia coli 25992 (ATCC), Salmonella choleraesuis 10708 (ATCC), Enterobacter aerogenes 13048 (NCDC), Klebsiella pneumonia 138 (NCDC), Citrobacter freudenii ATCC 43864, Yersinia enterocolitica ATCC 23715 and Serratia marcescens 13880 (ATCC) in respective growth medium for agglutination activity.21,22
Scanning electron microscopic study of agglutination
The selected WGA lectin was further studied for agglutination with L. monocytogenes using scanning electron microscopy (SEM)) using the method described by Facinelli et al.21 After the agglutination reaction, aliquots of samples (5–10µl) were air dried on glass cover slips and fixed in a solution of 2.5% glutaraldehyde in 75 mM phosphate buffer, (pH 7.4) for 45–60min. The fixed specimen was rinsed three times each for 15 min, in 75 mM phosphate buffer (pH 7.4). After the rinsing, samples were serially dehydrated in ethanol concentrations of 30, 50, 70, 80, 90 and 100% each for 15 min, respectively. After this step, the sample was air dried, and the specimen sample was mounted on a stub followed by gold coating. In gold coating, samples were encrusted with gold at approximately 100–200 Å thickness using an ion coater (Hitachi IB–3, Tokyo, Japan). The ion current was kept at 6–8mA with a fine vacuum of 0.05–0.07 torr for 2–4 minutes. The coated samples were then visualized using a SEM at 7000–30,000 X magnifications (EVO® 18, Carl ZEISS Special Edition, Cambridge,–UK).
Effect of sugars on lectin banding activity of L. monocytogenes
Different sugars such as D–glucose (Glc), D–galactose (Gal), D–mannose (Man) and N–acetylglucosamine (NAGA) were screened for sugar specificity of selected lectins with L. monocytogenes, using an inhibition assay.21,22 Different concentrations of sugar (0.1 to 1 M, 50µl) were mixed with 50µl of each selected lectin followed by incubation for 1 hr at 37oC. After incubation, the mixture was studied for agglutination using L. monocytogenes.21,22 Further, the percentage inhibition of agglutination activity was calculated by the ratio of agglutination activity lectin in the presence of the sugar to the agglutination observed with L. monocytogenes without addition of sugar.
Screening of lectin for the specific binding with L. monocytogenes
Among ten lectins screened for agglutination activity with different strains of L. monocytogenes cited above, HPA and WGA showed 80–90% activity with L. monocytogenes cells, having 8.38 log counts per mL, followed by 60–70% activity with AHL, 40–50% activity with UEL, LCL and SNA; 10–20% activity with PAL and MAL, respectively as shown in Figure 1. However, <10% agglutination was observed with PSA. Figure 2 depicts the microscopic observation of agglutination of L. monocytogenes with different lectins by simple staining technique and analysis through EMCCD. Maximum agglutination with all strains of L. monocytogenes was observed with WGA followed by HPA, AHA, UEL, MAA, LCL, PAL, SNA, CON A and PSA with an HA of 84.4%, 70%, 51.25%, 39.375%, 31.25%, 30.625%, 26.25%, 18.75%, 16.25% and 9.375%, respectively. The maximum HA was observed with WGA in the presence of different strains of L. monocytogenes; therefore, WGA was selected for further screening study with Gram positive and Gram negative bacteria. L. monocytogenes could interact with HPA and WGA whereas, Arachis hypogaea (PNA), Glycine max (SBA), and Vicia villosa (VVA) lectins failed to agglutinate L. monocytogenes 15313 or any of the other test strains under the experimental conditions.21 Another study conducted by Facinelli et al.23 observed that 26 strains of L. monocytogenes 15313 were agglutinated by WGA, 17 by Griffonia simplicifolia–1 (GS–1) by RCA–I (ricinus communis agglutinin), and nine by HPA. Therefore, WGA from TVA was selected for further study.
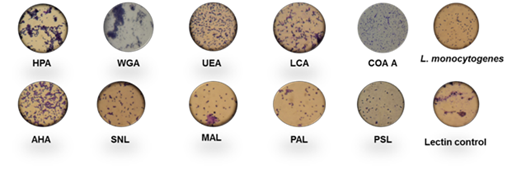
Figure 2 Light microscopic observation of agglutination of L. monocytogenes 15313 with different lectins by crystal violet staining. Lectin control contains 200μg/ml lectin dissolved in 10mM phosphate buffer, pH 6.8.
Screening of gram positive and gram negative bacteria for Lectin agglutination
All ten lectins including WGA were also further checked for agglutination activity with GPB (other strains of Listeria spp. such as Listeria innocua 33090, Listeria ivanovii 19119, Enterococcus faecalis, Bacillus cereus and Staphylococcus aureus)and GNB (E. coli O157:H7 Escherichia coli 25992 (ATCC), Salmonella choleraesuis 10708 (ATCC), Enterobacter aerogenes 13048 (NCDC), Klebsiella pneumonia 138 (NCDC), Citrobacter freudenii ATCC 43864, Yersinia enterocolitica ATCC 23715 and Serratia marcescens 13880). In our study, no or <10% HA activity was observed with WGA among GPB as shown in Figure 3. Figure 4 depicts the WGA agglutination activity in GNB wherein, zero agglutination was observed among E. coli, E. coli O157:H7, Enterobacter, and Serretia whereas, in the case of Salmonella and Klebsiella HA was 10–20% followed by 45% activity in Citrobacter. Jebor et al.24 found HA of PSA immobilized with glutaraldehyde in presence of Bacillus, Salmonella, Staphylococcus, Streptococcus and Pseudomonas as compared to E. coli, Enterobacter on the basis of increase in optical density at 660nm and through microscopic examination using Gran staining technique. In an another study by Payne et al.25 wherein they immobilized Helix pometia, Canavalia ensiformis, Agaricus bisporus and Triticum vulgaris i.e., WGA on magnetic microspheres for the evaluation of binding activity with L. monocytogenes, S. aureus, Salmonella and E. coli and observed 87–100% agglutination activity with L. monocytogenes in the presence of WGA, >92% binding with S. aureus in the presence of HPA, but poor binding with GNB in the presence of WGA and HPA. The membrane of GNB has not shown increased reactivity to WGA when exposed to increased salinity of the reaction mixture due to loosening of the rigid structure and it has improved the staining with the lectin.26 Based on these results, it was confirmed that the WGA was highly selective for L. monocytogenes. Therefore, WGA was selected for further study.
Scanning electron microscopic study of agglutination
Figure 5a & b shows the arrangements of the L. monocytogenes ATCC 15313 in the presence (Figure 5a) or absence of (Figure 5b) 200µg/ml of WGA lectin. Figure 5c shows the pure WGA lectin without L. monocytogenes ATCC 15313 cells. The electron microscopic observations depict that the control cells of L. monocytogenes ATCC 15313 cells were not induced to aggregate but they arranged in singles and pairs. However, the L. monocytogenes ATCC 15313 cell aggregation in the presence of lectin appeared to be gapped and patchier, but, cell rupture was only observed in some cases (as shown in Figure 5).
Inhibition of lectin binding activity of L. monocytogenes by different sugars
Carbohydrate inhibition studies were performed with L. monocytogenes 15313 using Glc, Gal, Man and NAGA. The WGA was consistently inhibited in presence of NAGA with an inhibition % of 0, 35, 50, 80 and 100% inhibition of WGA binding activity at 0.1, 0.25, 0.5, 0.75 and 1M concentration, respectively. In case of glucose, the % inhibition was 0, 25, 35, 55 and 100 and was observed at 0.1, 0.25, 0.5, 0.75 and 1M concentration, respectively. Whereas, in the case of galactose, the inhibition was only observed at 0.75 and 1M concentration with 75 and 90% inhibition respectively, the least inhibition was observed with mannose where only 25% inhibition was found at 1M concentration (Figure 6). The maximum or complete inhibition was observed in presence of NAGA, and indicates that the binding of L. monocytogenes cells to WGA lectin was highly specific for NAGA followed by glucose, galactose and mannose. The binding of L. monocytogenes cells to WGA was partially inhibited by glu and gal, suggesting that glu and gal moieties are also present on the cell surface structure of L. monocytogenes. The mannose inhibition of WGA agglutination was supported by Neu et al.27 that wide range of carbohydrates such as glu, gal, man, arabinose, rhamnose, etc. can inhibit the WGA binding in biofilm and about 31% inhibition in WGA binding of biofilm were seen under florescent microscopes using FITC–labelled WGA staining of biofilm. This work is supporting our claim of mannose inhibition of WGA binding with L. monocytogenes. Volf et al.28 & McGreal et al.29 reported that carbohydrate binding specificity is an important factor in the recognition of carbohydrates on pathogen cell surfaces. Further, Loessner et al.30 has shown that WGA lectin requires terminal NAGA residues present on the teichoic acid of the bacterial wall (WTAs) for attachment. Nir Paz et al.31 described the binding of fluorescently labeled WGA, a lectin that specifically binds terminal NAGA residues in cell wall polymers using Alexa Fluor 594®. They found that WGA was able to bind wild strains of L. monocytogenes such as 10403S and DP–L5415.
In our present study, L. monocytogenes has shown strong agglutination activity (HA) with WGA followed by HPA, AHA, UEL, MAA, LCL, PAL, SNA, CON A and PSA. The GPB have also shown HA with all lectins screened except WGA wherein HA was <10%. The GNB, especially Enterobacteriaceae groups, haven’t shown HA in presence of WGA except Citrobacter (45%), Klebsiella and Salmonella (10–20%) and maximum HA was observed in presence of ConA. The WGA activity with L. monocytogenes was also well established with SEM and by sugar inhibition assays. Based on the inhibition test, it was clear that WGA was specifically bound with NAGA followed by Glc, Gal was present on the cell wall of L. monocytogenes and whereas in case of man it was only 25% inhibition. From the above results, it is clear that WGA may be used as a marker protein for the detection of L. monocytogenes in milk and milk products.
This work was supported by the National Agriculture Innovation Project (NAIP), Niche area of Excellence (NAE), Outreach Programme (ICAR). The authors express their appreciation to personnel to Dr. S. K. Tomar, Principal Scientist & Incharge, SEM lab and Dr. Vaibao Lule Patil, PhD scholar, Dairy Microbiology Division for SEM study of lectin agglutination with L. monocytogenes. Director and Vice Chancellor, ICAR–National Dairy Research Institute (NDRI), Deemed University, Karnal for providing necessary research facility for conducting research work on Lectin binding of L. monocytogenes.
None.

©2017 Raghu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.