Journal of
eISSN: 2373-437X


Research Article Volume 4 Issue 3
1Faculty of Medical Technology, University of Zawia, Libya
2Faculty of Veterinary Medicine, University of Tripoli, Libya
3University of Nottingham, UK
Correspondence: Altayeb Elazomi, Faculty of Medical Technology, University of Zawia, Libya
Received: February 20, 2016 | Published: March 17, 2017
Citation: Elazomi A, Rahman MEA, Dhawi A, Liddell S, Lovell M, et al. (2017) Salmonella Enteritidis’ Vaccine Produces In Vitro and In Vivo Protection against Colonization. J Microbiol Exp 4(2): 00112. DOI: 10.15406/jmen.2017.04.00112
Salmonella entericcan be considered as one of the most important causes of food- poisoning with poultry thought to be the main source. Although S. Typhimurium, S. Enteritidis and the vast majority of other Salmonella serovars generally produce little systemic disease in adult chickens, they are able to colonize the alimentary tract of poultry. The two caeca are the main sites of the colonization of Salmonellae in chickens, and the bacteria can be easily harvested from the caeca for analysis. Bacterial proteins analyses utilizing SDS-PAGE showed differences between in vitro and in vivo that out of about 40 protein bands of in vitro preparation only a few (3-5) bands can be visualized from in vivo preparations. We suggested that some avian proteases might be responsible. Accordingly, and to investigate the hypothesis that an inactivated vaccine harvested from chickens is thought to be more protective than bacteria grown in broth culture, the immunogenicity of inactivated vaccines, either proteins or formalin killed bacteria (S. Enteritidis) harvested from chicken intestine and those from broth culture (in vitro), were compared using bacterial proteins or formalin killed bacteria as an orally inoCulated vaccine candidate in chicken. The results demonstrated that sonicated proteins extracts in general have a stronger effect as vaccines against S. Enteritidis colonization in chickens than formalin killed bacteria. In contrast, the in vitro sonicated proteins obtained from a nutrient broth culture had a much better protective vaccine effect than the in vivo sonicated proteins preparations harvested from bacteria grown in chickens.
Many bacterial pathogens such as Clostridium, StaphyloCoCcus, Campylobacter and many other bacterial strains are capable of causing food-poisoning, and Salmonella enterica can be considered as one of the most important causes with poultry thought to be the main source. Although S. Typhimurium, S. Enteritidis and the vast majority of other Salmonella serovars generally produce little systemic disease in adult chickens, they are able to colonize the alimentary tract of poultry, resulting in contamination of poultry carcasses and entry into the human food chain. However, there is a great demand to control food-poisoning salmonellosis at both breeder and layer levels at the national and global level in order to produce Salmonelle-free poultry products, due to the current correlation between S. Enteritidis PT4 and poultry products. Salmonellosis costs the European Union a minimum of 500-900 million Euros annually. Salmonellosis in food animals is a major target for reduction of human infection by the European Union. Legislation has been introduced to monitor the most important Salmonellae serovars. The major Salmonellae serovars of public health consequence are S. Typhimurium and S. Enteritidis (causing 15% and 60% respectively of all cases in Europe in 2002).
Elazomi and Dhawi studied newly-hatched chickens infected with S. Enteritidis. They analyzed proteins of S. Enteritidis in the caeca of 1-day old checks (in vivo) together with a comparison with nutrient broth medium (in vitro) in order to detect changes in the pattern of protein expression during infection. The preliminary exploratory study of individual bands identified major proteins (flagellin of S. Enteritidis and Typhimurium fliC) and mixtures of proteins including 60kDa chaperonin groEL and glyceraldehyde-3-phosphate dehydrogenase gap A. Some proteins may be expressed equally both in vivo and in vitro (e.g. fimbrial , flagellar, outer membrane protein, metabolic , regulatory, and LPS-synthesis encoded genes). These proteins are predicted to play a major role in colonization. Chicken caecal colonization by paratyphoid Salmonella (e.g. Enteritidis, Typhimurium and others) has been linked to the physical attachment by fimbriae1 motility,2 type three secretion system (T3SS) of Salmonella Pathogenicity Islands “SPI-1 and SPI-2”,3 bacterial cell wall component lip polysaccharide “LPS”4,5 and outer membrane proteins “OMPs”.6 This comparison showed differences between the two profiles and indicated that it is difficult to make a reasonable comparison as out of about 40 protein bands of in vitro preparation only a few (3-5) bands can be visualized from in vivo preparations, the reason behind that thought to be the degradation of in vivo protein with some avian proteases.
Then we hypothesized that inactivated vaccines prepared from bacteria grown in vivo in chickens will give better protection than a vaccine prepared from bacteria cultured in vitro because they will be expressing antigens normally expressed during infection/colonisation. Accordingly, and to investigate the hypothesis that an inactivated vaccine harvested from chickens is thought to be more protective than bacteria grown in broth culture, the immunogenicity of inactivated vaccines, either proteins or formalin killed bacteria (S. Enteritidis) harvested from chicken intestine and those from broth culture (in vitro), were compared using bacterial proteins or formalin killed bacteria as an orally inoCulated vaccine candidate in chicken.
Preparation of formalin-killed bacterin from bacterial
Cells cultured in vitro in nutrient broth
A single colony of S. Enteritidis PT4 (antibiotic sensitive parent strain) was inoCulated into 10ml NB and incubated overnight at 37 °C and 1ml of this broth culture was transferred into 2 x 100ml NB in 250ml flasks and incubated for two hours at 37 °C in a shaking incubator at 200rpm. Each flask was then decanted to three 50ml Falcon tubes each containing 33.3ml, the tubes then centrifuged at 5000g for 30min at 20°C and the supernatants were discarded without disturbing the pellets. Subsequently the pellet from each tube was re-suspended with 3.33ml NB, then the content of three Falcon tubes arising from the same flask were mixed into one Falcon tube. The contents (10ml NB 109 bacterium/ml) which is equivalent to 108/0.1ml=3x108 bacterium/0.3ml = 5x108/0.05ml which used for chicken injection (i.m) to both breast sides.
Preparation of formalin-killed bacterin from bacterial
Cells cultured in vivo in chicken caeca
90 newly hatched chickens were inoCulated orally with S. Enteritidis PT4 (antibiotic-sensitive parent strain) culture (1x106 cell). On the following day the chickens were killed and the infected caecal contents were collected. Caecal contents from three of the chickens were collected separately for viable count estimations and purity checking. Caecal contents from the remaining birds were collected together in two Falcon tubes in dry ice (1 tube each day as the collection was made over two separate days (from 15 and 40 birds respectively) and used for this vaccine preparation. The first tube that contained the caecal contents of 15 birds contained 0.936g and 2.5x1010 cfu/ml and tube two that contained the caecal contents of 40 birds contained 4.652g and 1x1011 cfu/ml; so the total weight was 5.5gm in 5.5ml. Therefore, the total bacterial number was 1.25x1011 bacteria. Both tubes were diluted together in 12.5ml PBS and mixed well and 3ml were collected from them and added to 27ml of NB for formalization. Each sample was diluted to thus contain 1x109cfu/ml and was stored at -80° C until used.
Formalization of S. Enteritidis killed vaccine
To each bottle of 10ml suspension of bacteria 0.2ml of 40% formaldehyde was added and vortexed for 30s and divided equally between 10 x 1ml sterile tubes (Eppendorff) and stored in a cold room at 4 °C until needed.
Preparation of sonicated protein vaccine
Whole cell proteins were prepared from S. Enteritidis bacteria harvested from chickens or from a NB culture.
Preparation of in vivo S. Enteritidis protein vaccine
A total of 100 newly hatched chickens were inoCulated orally within 18 h of hatching. Chickens were infected orally with 0.1 ml of a culture of the antibiotic-sensitive parent S. Enteritidis PT4, grown for 16 h in nutrient broth at 37ºC and diluted in sterile nutrient broth to contain 107 cfu/ml. After 16-18 hs post-infection chickens were killed one-by-one, and the caecal contents were harvested from both chicken caeca of each bird. The caecal contents of three randomly chosen chicks were transferred to three separate sterile universal tubes which were placed on ice to test for purity and viable bacterial number estimation on MacConkey agar and nutrient agar. The caecal contents of the remaining chickens were put in 50 ml Falcon tubes in dry ice and then were stored at -80oC until needed. Three chicks were left without inoCulation and their uninfected caecal contents were used to streak on MacConkey agar and nutrient agar plates and incubated for overnight at 37ºC to ensure that there were no contaminants with other bacteria. For the vaccine preparations the S. Enteritidis-infected caecal contents were diluted in nutrient broth and then centrifuged at 20,000xg for 5 min at 4ºC (Avanti®J-E Beckman centrifuge coulter), then the supernatant was discarded and the pellets were resuspended in NB, followed by sonication (Sonics VCX500) for 5 min immediately after adding the protease inhibitors (Sigma P8465). This sonicates was then centrifuged at 15,000 g for 10 min at 4oC. Subsequently, the supernatant was filtered using 0.45 μm filters and stored in 1ml aliquots in Eppendorff tubes at -20oC until required.
Preparation of in vitro S. Enteritidis protein vaccine
A single colony of the parental S. Enteritidis PT4 sensitive strain was picked and used to inoCulate 10ml NB in a universal bottle which was then incubated overnight at 37oC. On the following day 250ml flasks, each containing 100ml nutrient broth, were inoCulated with 1ml of the overnight broth culture of S. Enteritidis PT4 and incubated overnight at 37oC shaking incubator (150 rpm). The contents of these broth cultures were divided into four 50ml centrifuge tubes and centrifuged at 20,000 x g for 5 min at 4ºC (Avanti®J-E Beckman centrifuge coulter).The pellet from each tube was resuspended in 5ml NB and sonicated for 5 min (Sonics VCX500)) after adding the protease inhibitors (Sigma P8465), followed by centrifugation at 15,000xg for 10 min at 4oC and filtration as mentioned above. The proteins preparations were then stored at -20oC until required.
Vaccine Quality Control
Protein sonicates harvested from both in vivo and in vitro environment were streaked on MacConkey and nutrient agar plates which were incubated overnight at 37°C to chick for any Salmonella growth, this also applied for the formalized bacteria.
First vaccination experiment
A lot of 100 1-day commercial layer chickens obtained from Millennium Hatchery Hy-Line UK Ltd (Studley Warwickshire), were utilized in this experiment. On the day of arrival birds were divided into five groups each of 20 birds with each group being placed in separate rooms which were cleaned down with a mixture of water and disinfectant (Trigene Disinfectant 20L Clear from Scientific Laboratory Supplies Ltd (CLE1320). Followed by chemical fogging with Virkon disinfectant from Sigma (Z692158), this allowed to rest for more than 24h and rinsed off. Chicks were distributed between the rooms as follows (in vivo formalin-killed – Room I; in vivo sonicated proteins – Room II; in vitro formalin-killed – Room III; in vitro sonicated protein – Room IV; unimmunized – Room V). All chickens were kept on solid flooring covered with wood straw and were provided with food and sterile drinking water. Subsequently, all birds in all groups were inoCulated with 0.1 ml of neat Avigard gut microflora (Microbial Developments Limited, UK), then at the fifth day of age all chickens were inoCulated intramuscularly (i/m), into the breast muscle, with 0.05ml containing either formalin-killed bacterial cells or protein preparation. Chickens were also inoCulated orally with 0.1 ml of the corresponding vaccine for each group as shown in Table 1. The remaining group number five (control) was inoCulated with sterile NB. At three weeks of age the vaccination program was repeated with all birds inoCulated with 0.3 ml orally and 0.1 ml i/m using the corresponding vaccine for each group. All birds were challenged with 0.5 ml of NB culture (3 x 108 cells) of a nalidixic acid resistant (NalR) mutant of S. Enteritidis strain at week 5 of their age. Cloacal swabs were collected from all birds at 1st, 2nd, 3rd, 4th, 7th, 14th, 21st and 28th day post- challenge for a semi-quantitative estimation of bacterial shedding [7,8] of the challenge S. Enteritidis NalR by plating on BG agar supplemented with nalidixic acid (20µgm/ml-1) and novobioCin (1µgm/ml-1). On day 28 post-infection after collection of cloacal swabs all birds were slaughtered and their caecal contents were collected for a semi-quantitative S. Enteritidis NalR count estimation.
ay/Group |
Group I |
Group II |
Group III |
Group IV |
Group V |
1 |
0.1ml Avigard orally |
0.1 ml Avigard orally |
0.1ml Avigard orally |
0.1ml Avigard orally |
0.1 ml Avigard orally |
5 |
0.05ml in vivo killed bacteria i/m |
0.05 ml in vivo protiens i/m |
0.05ml in vitro killed bacteria i/m |
0.05ml in vitro proteins i/m |
0.05ml sterile NB i/m |
0.1ml in vivo killed bacteria orally |
0.1 ml in vivo proteins orally |
0.1 ml in vitro killed bacteria orally |
0.1ml in vitro proteins orally |
0.1 ml sterile NB orally |
|
21 |
0.1ml in vivo killed bacteria i/m |
0.1 ml in vivo proteins i/m |
0.1 ml in vitro killed bacteria i/m |
0.1ml in vitro proteins i/m |
0.1 ml sterile NB i/m |
0.3ml in vivo killed bacteria orally |
0.3 ml in vivo proteins orally |
0.3ml in vitro killed bacteria orally |
0.3ml in vitro proteins orally |
0.3 ml sterile NB orally |
|
35 |
All groups were challenged with 0.3ml contains (5X108) S. Enteritidis orally |
||||
Cloacal swabs |
Cloacal swabs were collected from each birds in all groups for Salmonella count at day 1,4,7, 14, 21 and 28 post challenge |
||||
Caecal contents |
All birds were killed at week nine of their age and caecal contents were collected from each bird in all groups for Salmonella count |
||||
Table 1 Vaccine administration regime and sample collection for vaccination experiment one (oral challenge).
Second vaccination experiment
This experiment was different from the first experiment only in the route of challenge and types of sample collected. The birds and groups were identical to those in the first experiment. As in experiment 1 all birds in all groups were inoCulated with 0.1 ml of neat Avigard gut microflora (Microbial Developments Limited, UK), followed by intramuscular and oral vaccination as before as shown in Table 2. When birds were 3 weeks of age the vaccination program was repeated as before. Subsequently, all birds were challenged intravenously via the wing vein with 0.1ml (1x106 cells) of S. Enteritidis NalR at 5 weeks age. Five birds from each group were selected randomly and killed at 1, 4, 6 and 8 days post-challenge. Immediately after killing each bird was sprayed with 70% ethanol and then spleen and liver samples were collected in pre-labelled, pre-weighed sterile universal bottles using separate sterile scissors and forceps. During sample collection all organs sampled were observed for any clinical signs. The caecal contents for each bird were then collected separately in pre-weighed sterile universal bottles. The three bottles for each bird were kept on ice prior to reweighing and diluting in x 9 the weight of the sample in PBS. All tissue samples (liver and spleen) were kept on ice until they weighed and then proportional amounts (10xweight expressed as volume) of PBS (pH 7.2) were added into each tube. Each tissue portions was homogenized in a Griffiths tubes in PBS (pH 7.2) to obtain homogenous suspension [2] prior to dilution for counting. This together with ax9 dilution of the caecal contents were used for bacterial count estimations.
y/Group |
Group I |
Group II |
Group III |
Group IV |
Group V unvaccinated control |
1 |
0.1 ml Avigard orally |
0.1 ml Avigard orally |
0.1 ml Avigard orally |
0.1 ml Avigard orally |
0.1 ml Avigard orally |
5 |
0.05 ml in vivo killed bacteria i.m |
0.05 ml in vivo protiens i.m |
0.05 ml in vitro killed bacteria i.m |
0.05 ml in vitro protiens i.m |
0.05ml sterile NB i.m |
0.1 ml in vivo killed bacteria orally |
0.1 ml in vivo proteins orally |
0.1 ml in vitro killed bacteria orally |
0.1 ml in vitro proteins orally |
0.1 ml sterile NB orally |
|
21 |
0.1 ml in vivo killed bacteria i.m |
0.1 ml in vivo proteins i.m |
0.1 ml in vitro killed bacteria i.m |
0.1 ml in vitro proteins i.m |
0.1 ml sterile NB i.m |
0.3ml in vivo killed bacteria orally |
0.3 ml in vivo proteins orally |
0.3ml in vitro killed bacteria orally |
0.3ml in vitro proteins orally |
0.3 ml sterile NB orally |
|
35 |
All birds in all groups Challenged intravenously with 0.1 ml containing (1x106) Live S. Enteritidis |
||||
Post challenge sample collections |
Five randomly selected birds from each group were killed at day 1, 4, 6 and 8 post infections, tissue portion of their spleen and liver plus caecal contents were collected for Salmonella count. |
||||
Table 2 Vaccine administration regime and sample collection for vaccination experiment two (intravenous challenge)
Bacterial Culture and Count
Enumeration of bacteria in chicken faeces (Experiment I)
After collection of all swabs 2ml selenite broth (Oxoid, CM0395) were added to each tube, followed by brief vortexing. Once they were mixed, each swab was placed in a standard manner on brilliant green agar plate (BGA) supplemented with nalidixic acid (20µgm/ml-10) and novobioCin (1µgm/ml-1). BGA in a standard manner.9 The inoCulated plates and the selenite broths were incubated overnight at 37°C. Then the swabs were left into selenite broth tubes for overnight incubation at 37°C prior to plating on BGA, to encourage the growth of Salmonellae and inhibit the growth of other flora. Then the overnight incubated swabs were plated again on the antibiotic-containing BGA media and incubated overnight at 37°C. Plates inoCulated directly were read and observed for Salmonella growth using a semi-quantitative estimation of faecal shedding and caecal colonization of Salmonella from infected chickens.1,7,10-12 Next day the enrichment plates were also checked for Salmonella growth. Xylose Lysine Deoxycholate (XLD) media (Oxoid, CM0469) was used as a confirmatory test for any Salmonella growth. Suspect colonies were sub-cultured on this media and incubated overnight at 37°C, and the plates were checked for black colonies indicating Salmonella as a result of H2S production, in addition to slide agglutination tests.
Bacterial enumeration in tissues samples (Experiment II)
The bacterial count of S. Enteritidis NalR in spleen, liver and caecal contents for the 5 birds of each group (at day 1, 4, 6 and 8 post challenge), were estimated by serial dilution and plating aliquots of dilutions.9 Aliquots of each dilution were plated on BGA plates supplemented with nalidixic acid (20µgm/ml-1) and novobioCin (1 µgm / ml-1) and incubated overnight at 37°C. Bacterial colonies were counted and the viable count converted into Log10 numbers. The Xylose lysine Deoxycholate medium XLD (Oxoid, CM0469) and slide agglutination tests were also used as confirmatory test to confirm any Salmonella growth.
Data Analysis
Analysis of data obtained from experiment I
Cloacal swabs were taken from each bird two days previous to challenge inoCulation for culture to guarantee that the chicks are free from Salmonellae. Differences in percentage excretion rates between groups of birds were compared using χ2, and this was considered as statistically significant if the P value was (<0.05).
Analysis of data obtained from Experiment II
As in experiment I Cloacal swabs were taken from each bird two days before being challenged for culture to guarantee that the chicks are free from Salmonellae. The bacterial counts of S. Enteritidis NalR (challenge) of the tissues (spleen and liver) and caeca in different groups on BGA plate, in different time points were recorded and the P value of each group compared to the control group were calculated using Student’s unpaired t test (Microsoft Office 2007). A P value of (< 0.05) was considered as statistically significant.
It was decided to carry out experimental in vivo infection using 1-day old chicks primarily to avoid the development of intestinal microflora, which would be likely to have a significant effect on interference in interpreting the patterns of protein expression in S. Enteritidis as well as to enable the bacterium of interest (S. Enteritidis) to multiply extremely well in the absence of competitive colonizers.2,13 Using birds aged from 2-6 weeks is the best model to study Salmonella colonization of chicken, as their gut flora is mature (Barrow, personnel communication), but for studying Salmonella proteins this might give a false results due to cross contaminations of gut flora. The model used therefore, uses colonization during infection in newly-hatched chickens and which might oCcur in a hatchery. Interestingly, a study of Campylobacter jejuni gene transcription using a similar model revealed a similarity between this model and birds that had already established their flora.13 On the other hand, other researchers have used a different model (spectinomycin-treated mouse) with E. coli.14,15 So there is good reason to suppose that the protein expression of Salmonella in newly hatched chickens may also be similar to that in old chicks with established flora. In addition, the caeca empty and fill several times a day, the phases of growth of the inoCulated bacteria in individual birds may be different. However, it will be clear that major differences in transcription between in vivo and in vitro culture will be identified. A protein analysis of S. Enteritidis in the caeca of 1-day old checks (in vivo) together with a comparison with nutrient broth medium (in vitro) was used to detect changes in the pattern of protein expression during infection and in particular to identify proteins that enable this strain to colonise the caeca. It was decided to use S. Enteritidis proteins extracted from stationary phase as the protein concentration of mid log phase was very low. The problems assoCiated with harvesting in vitro grown cultures also apply, particularly relating to a synchronicity of growth. The issues assoCiated with cultures of this sort are that (i) bacterial growth is asynchronous. However, bacterial growth in vivo will also be asynchronous, and (ii) the relevance of using a rich nutrient broth culture as a control can be questioned. However, there is no truly rational way of establishing a control for in vivo growth and previous work,16 indicates that our approach is an appropriate approach. We compare the immunogenicity of bacteria (S. Enteritidis) harvested from the intestine with those grown in vitro in nutrient broth cultures. The preparations would include (i) formalin-killed whole bacteria harvested from the caeca, (ii) whole cellular proteins prepared from in vivo-cultured bacteria and (iii) in vitro-grown bacteria in NB (iv) whole cellular proteins prepared from in vitro-grown bacteria in NB all of which would be tested for their ability to protect against Salmonella colonization in chicken.
Quality control of the vaccine
No growth was detected after culturing the formalized S. Enteritidis PT4, as well as protein sonicates harvested from both in vivo and in vitro environments on MacConkey and nutrient agar plates for overnight at 37 °C.
Results for experiment I (orally challenged chicks)
No Salmonella organisms were isolated from the chickens on receipt. The percentage excretion rates of the challenge Salmonella strain in the different groups are shown in Table 3. When Salmonella was cultured by direct plating if the colony numbers present per plate was 1 or more this was designated as ≥1, while when they were 50 colonies or more this was designated as ≥50.17 The bacteria cultured by enrichment followed by plating were shown as the percentage of positive swabs, which had been confirmed by XLD agar and slide agglutination tests as shown in Table 3.
Percentage of chickens (20 birds per group) excreting S. Enteritidis nalr (challenge strain) from direct plates and number of positive birds (Positivity %) from enriched plates at different time points post-infection |
||||||||||||||||
Sample |
Days PI |
In Vivo Killed Bacteria |
In Vivo Proteins |
In Vitro Killed Bacteria |
In Vitro Proteins |
Unvaccinated |
||||||||||
Direct |
Enriched |
Direct |
Enriched |
Direct |
Enriched |
Direct |
Enriched |
Direct |
Enriched |
|||||||
Cloacal swabs |
≥ 50 |
≥ 1 |
Birds( No& %) |
≥50 |
≥1 |
Birds( No& %) |
≥ 50 |
≥ 1 |
Birds( No& %) |
≥50 |
>1 |
Birds( No& %) |
≥ 50 |
≥ 1 |
Birds( No& %) |
|
1 |
0% |
0% |
13 (66 %) |
0% |
10% |
10(52%) |
5% |
33% |
9 (43 %) |
0% |
16% |
5(26%) |
0% |
20% |
9 (45 %) |
|
4 |
0% |
24% |
9 (43 %) |
14% |
38% |
16(81%) |
0% |
10% |
11 (57 %) |
0% |
16% |
5(26%) |
0% |
25% |
10 (52 %) |
|
7 |
0% |
29% |
10 (52 %) |
5% |
5% |
7(33%) |
0% |
5% |
12 (62 %) |
0% |
0% |
6(32%) |
5% |
5% |
10 (50 %) |
|
14 |
0% |
0% |
6 (29 %) |
0% |
0% |
3(14%) |
5% |
5% |
4 (19 %) |
0% |
0% |
0(0%) |
5% |
10% |
3 (15 %) |
|
21 |
0% |
0% |
4 (19 %) |
0% |
0% |
2(10%) |
0% |
0% |
3 (14 %) |
0% |
0% |
0(0%) |
5% |
15% |
4 (20 %) |
|
28 |
0% |
0% |
3 (14 %) |
0% |
0% |
2(10%) |
0% |
0% |
2 (10 %) |
0% |
0% |
0(0%) |
0% |
15% |
4 (20 %) |
|
Caecal content |
28 |
0% |
0% |
0(0%) |
0% |
0% |
0(0%) |
0% |
0% |
0(0%) |
0% |
0% |
0(0%) |
5% |
20% |
7 (35 %) |
Table 3 Effect of vaccinating with formalin-killed S. Enteritidis and whole-cell sonicated protein preparation on faecal excretion of S. Enteritidis NalR (challenge strain),results obtained from direct plates; plus results of BGA enriched plates shown number of S. Enteritidis NalR positive birds (positivity %), from cloacal and caecal sample collected at different time points post infections, chicks were orally inoculated.
Based on evaluation of the results of all samples collected (either caecal contents or cloacal swabs), the percentage of chickens positive for S. Enteritidis NalR challenge strain for the first day post-infection was 66% and 43% for the birds in group one and three which were treated with formalin killed bacteria harvested from in vivo and in vitro environment respectively, whereas the percentage of faecal excretion of the challenge strain in group two and four which were treated with in vivo and in vitro sonicated proteins preparation respectively were 52% and 26%, compared with 45% in untreated control (group five). There were a variable change of these percentages at 4th day post infection as the percentage remained same (26%) in group four which had been treated with in vitro sonicated proteins while in group one, two and three the percentages were 43%, 81% and 57% respectively, with 52% in the control group. Subsequently, one week post infection the corresponding values were 52%, 33%, 62% and 32% in treated groups from one to four respectively and 50% in the unvaccinated control group. Then there was a noticeable decrease in the percentages of faecal excretion in all groups two weeks post infection as the percentages were 29%, 14%, 19%, 0% and 15% in groups from one to five respectively. Moreover, three weeks post challenge the percentage of positive birds’ faecal excretion was 19 % for the in vivo killed vaccine group (group one) and 10% for the in vivo sonicated proteins (group two), and it was 14% for the in vitro killed vaccine group ( group three), while there was no faecal excretion (0%) in group four which was vaccinated with in vitro protein preparation with no difference from 2nd week post infection compared with (20%) of unvaccinated control group. There were no noticeable difference in faecal excretion in week four post infection with percentages 14%, 10%,10%, and 20% in groups from 1 to five respectively. Figure 1 below illustrate the percentages of chickens’ faecal exertion of S. Enteritidis NalR (challenge strain) at different time points (1st ,4th, 7th, 14th, 21st, and 28th) days post infection. In summary, both in vitro and in vivo protein preparations had a much greater immunization effect than that produced by killed bacteria. The P values were (χ2=28.3. P<0.001) and (χ2=16.77. P<0.001) for the in vitro and in vivo proteins treated groups respectively, which were considered as statistically significant. The in vivo protein preparation unexpectedly had a lower immunogenic effect than did the in vitro proteins preparation. From the caecal samples collected at week four post infection Salmonella was detected only in unimmunised control (group five), while no growth of any Salmonella were observed in all treated groups (<1x102cfu/ml) as shown in Table 3 above and Figure 1 below.
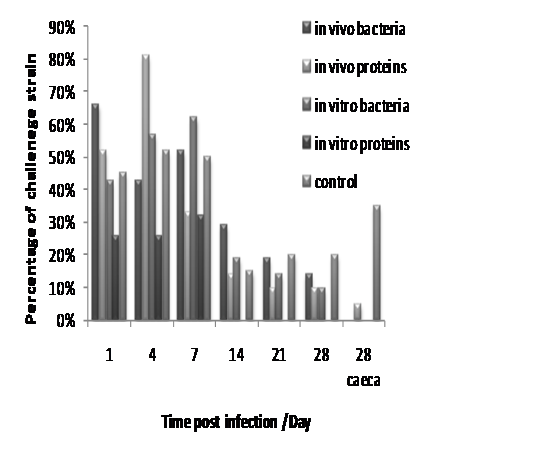
Figure 1 Faecal excretion of challenge S. Enteritidis strain following vaccination with formalin killed Salmonella harvested from chickens (in vivo) or nutrient broth (in vitro) plus Salmonella proteins produced from bacteria cultured either in chickens (in vivo) or in nutrient broth (in vitro) compared with unvaccinated control, this figure also shown that no growth of any Salmonella were detected in caecal contents of all treated groups 4 weeks post infection.
Results for experiment II (Intravenously challenged chicks)
The results presented in Table 4 shows the averages ofLog10 Salmonellae counts in liver and spleen of five chickens taken at different time points post-infection from the four groups of immunised birds plus the control group with the P values. No Salmonellae were detected in caecal contents of any bird from the group immunised with sonicated proteins, or groups treated with formalin-killed bacteria although some other bacterial growth such as E. coli, Klebsiella were observed as illustrated in Table 5 and Figure 4. Salmonellae challenge organisms were detected in the control group. Moreover, as shown in Figure 2 below when chickens were challenged intravenously with parent S. Enteritidis NalR, the viable counts (Log10) of Salmonellae in the spleen on the 1st day post-infection were 5.1, 5, 4.6 and 4.7 cfu/ml in group I, II, III and IV which were vaccinated with in vivo killed bacteria, in vivo proteins, in vitro killed bacteria and in vitro proteins respectively, whereas the count in unimmunised control group (V) was 4.8 cfu/ml. Surprisingly, the count of Salmonellae in spleen tissues on the 4th day post-infection in all immunised groups (Log 5.3, 5.2, 5.2 and 5.1 cfu/ml) respectively, were all higher than unimmunised control group which was Log 4.7cfu/ml, with only very small variations of Salmonellae count on the 6th day post infection 4.9, 4.8, 4.9 and 4.8 cfu/ml for the groups immunised with in vivo killed bacteria, in vivo proteins, in vitro killed bacteria and in vitro proteins respectively, compared with 3.1 cfu/ml in control group. There were no big differences observed in chicken spleen colonization with Salmonellae on the 8th day post challenge in all treated groups as well as unimmunised control group with bacterial count log10 4.5, 4.0, 4.9, 4.1 and 4.0 cfu/ml respectively. At 1 day post-infection the bacterial count in liver were Log 3.9 and 4.0 cfu/ml for the birds in group I and III, which were immunised with in vivo and in vitro formalin-killed bacteria respectively, whereas the Salmonellae count in groups two and four immunised with in vivo and in vitro proteins preparation were Log 3.8 and 4.0 cfu/ml. This result was unexpected as the counts in all vaccinated groups were again higher than that of unimmunised birds (Log 3.4 cfu/ml) as illustrated in Table 4 below. On this day the P value of Salmonellae in liver tissue in all vaccinated groups was P < 0.00, P < 0.07, P < 0.01 and P < 0.03 respectively compared to the unimmunised control. Salmonella countsin liver on 1st, 4th, 6th and 8th day post infection steadily decreased in all vaccinated and unimmunised birds as shown in Table 4 and Figure 3 below. Furthermore, the Log10 Salmonella counts in liver on the 4th day post infection were 3.6 and 3.2 in groups vaccinated with in vivo and in vitro killed bacteria respectively, while in both groups vaccinated with in vivo and in vitro proteins preparation the Log counts were 3.5 and 3.1 respectively compared with 2.3 in unimmunised birds, with P value (P < 0.08, P < 0.08, P < 0.18, P < 0.2) in the groups treated with in vivo killed bacteria, in vivo proteins, in vitro killed bacteria and in vitro proteins respectively. On day 6 post challenge the mean Salmonellae Log10 count was 2.6 in groups I and II which were inoCulated with either in vivo bacteria or in vivo proteins, while in groups III and IV, which were inoCulated with in vitro killed Salmonellae and in vitro proteins respectively, the Salmonellae Log10 count was 3.0 in both group. Consequently, however, on the last day of sample collection ( the 8th day post infection), the mean Log10 Salmonellae count in liver was 2.0 and 2.2 for in vivo and in vitro killed bacteria respectively, 2.06 and 1.0 for in vivo and in vitro protein vaccines respectively and 2.3 for unimmunised birds as shown in Figure 3 below. In addition, bacterial counting was performed on caecal contents for all birds and with the exception of some lactose fermentor bacteria cultured from different group birds’ caeca, no Salmonellae were detected (< Log 2 cfu/ml) in caecal contents of any bird.
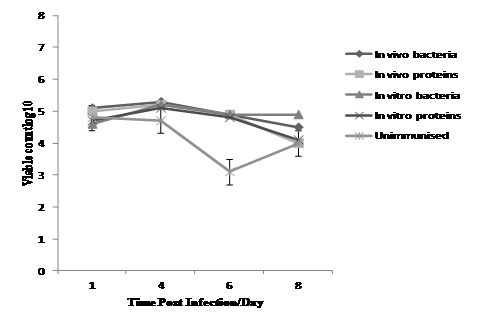
Figure 2 The number of Salmonella Log10 cfu/ml in chicken’s spleen tissue in the groups of birds (each of 20 birds) treated with either S. Enteritidis whole cellular in vivo and in vitro sonicated proteins preparation or in vivo and in vitro formalin killed S. Enteritidis compared with unimmunised control post challenge with parent strain (S. Enteritidis NalR) inoculated intravenously.

Figure 3 The number of Salmonella Log10 cfu/ml in liver tissue in the groups of birds treated with either S. Enteritidis whole cellular in vivo and in vitro sonicated proteins preparation or in vivo and in vitro formalin-killed S. Enteritidis compared with unimmunised control post challenge with parent strain (S. Enteritidis NalR) intravenously.
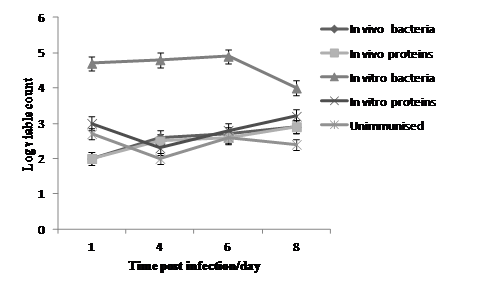
Figure 4 The number of lactose fermentor bacteria Log10cfu/ml-1 in caecal contents in the five groups of birds treated with in vivo or in vitro protein preparations of S.Enteritidis plus formalin killed bacteria harvested from in vivo and in vitro conditions compared with unimmunised group at 1st, 4th, 6th and 8th day post intra-venous infection with challenge strain S. Enteritidis NalR.
Days PI |
In Vivo Killed Bacteria |
In Vivo Proteins |
In Vitro Killed Bacteria |
In Vitro Proteins |
Unvaccinated |
|||||||||||||||||||||||||
Liver |
Spleen |
Liver |
Spleen |
Liver |
Spleen |
Liver |
Spleen |
Liver |
Spleen |
|||||||||||||||||||||
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
|
1 |
3.9 |
0.1 |
0 |
5.1 |
0.04 |
0.23 |
3.8 |
0.1 |
0.07 |
5 |
0.03 |
0.2 |
4 |
0.1 |
4.6 |
0.2 |
4 |
0.1 |
0.03 |
4.7 |
0.1 |
0.7 |
3.4 |
0.08 |
- |
4.8 |
0.15 |
- |
||
4 |
3.6 |
0.1 |
0.08 |
5.3 |
0.15 |
0.5 |
3.5 |
1 |
0.08 |
5.2 |
0.1 |
0.1 |
3.2 |
0.1 |
0.18 |
5.2 |
0.1 |
0.1 |
3.1 |
0.1 |
0.2 |
5.1 |
0.1 |
0.2 |
2.3 |
0.5 |
- |
4.7 |
0.19 |
- |
6 |
2.6 |
0.1 |
0.04 |
4.9 |
0.13 |
0.1 |
2.6 |
0.2 |
0.08 |
4.8 |
0.1 |
0.08 |
3 |
0 |
0.04 |
4.9 |
0.2 |
0.1 |
3 |
0.1 |
0.02 |
4.8 |
0.1 |
0.1 |
0.9 |
0.6 |
- |
3.1 |
0.48 |
- |
8 |
2 |
0 |
0.1 |
4.5 |
0.6 |
0.09 |
2.06 |
0.06 |
0.8 |
4 |
1 |
0.8 |
2.2 |
0.1 |
0.4 |
4.9 |
0.3 |
0 |
1 |
0.2 |
0.12 |
4.1 |
0.2 |
0.7 |
2.3 |
0.7 |
- |
4 |
0.13 |
- |
Table 4 The protective effect of formalin-killed bacteria and protein preparation from S. Enteritidis harvested from chickens in vivo or nutrient broth in vitromeasured by liver and spleen counts of chicks inoculated intravenously by the parent strain. Log10 mean viable counts of Salmonella per ml of homogenized liver tissue of 5 birds from each group/time poin
However, 1 day post infection birds vaccinated with in vitro formalin killed showed the highest bacterial count (Log10 4.7cfu/ml) of lactose fermentors in their caecal contents with P value (P<0.04), followed by the group immunised with in vitro proteins which produced counts of Log 3.0 cfu/ml with P value (P <0.6). On 4th day post-infection the birds immunised with in vitro bacteria again showed the highest count (Log 4.8 cfu/ml) followed by the groups immunised with in vivo bacteria and in vivo proteins which had mean counts of Log 2.6 cfu/ml and 2.5cfu/ml respectively as illustrated in Table 5 and Figure 4. Caecal contents that showed no bacterial growth were considered as (<Log 2cfu/ml) which means that the average of bacterial count is less than the limit of detection.
Days post infection |
In Vivo |
In Vivo Proteins |
In Vitro |
In Vitro Proteins |
Unvaccinated |
||||||||||
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
Log 10 |
SE |
P |
|
1 |
2 |
0 |
0.3 |
2 |
0 |
0.07 |
4.7 |
0.2 |
0.04 |
3 |
0.2 |
0.6 |
2.7 |
0.7 |
|
4 |
2.6 |
0.37 |
0 |
2.5 |
0.3 |
0.08 |
4.8 |
0.2 |
0 |
2.3 |
0.2 |
0.18 |
2 |
0 |
- |
6 |
2.7 |
0.28 |
0.9 |
2.6 |
0.2 |
0.08 |
4.9 |
0.2 |
0.01 |
2.8 |
0.2 |
0.64 |
2.65 |
0.4 |
- |
8 |
2.9 |
0.56 |
0.15 |
2.9 |
0.5 |
0.3 |
4 |
0.8 |
0.1 |
3.2 |
0.8 |
0.61 |
2.46 |
0.3 |
- |
Table 5 The effect of formalin-killed bacteria and protein preparation of S. Enteritidis harvested from in vivo and in vitro conditions on colonisation of chicken caeca with lactose fermentor bacteria when challenged intravenously by the parent strain S. Enteritidis NalR. (Average viable counts log10 per 1ml of caecal contents).
SE, Standard Error; P, P value.
Control of Salmonella infections in chickens is crucially important towards the aim of reduction of human food-poisoning salmonellosis. Legislation has been introduced by the European Union (Directive 2003/99/EC, Regulation 2160/2003) to monitor the most important Salmonella serovars with timetabled requirements for submission of action plans to control infections in major hosts, particularly pigs and poultry. As a part of this both live and inactivated vaccine are now used in many countries both in the EU and around the world. Nevertheless, live vaccines used in the EU are produced by chemical mutagenesis, and are antibiotic resistant.
In the present study four types of vaccine were produced from S. Enteritidis PT4. The vaccines were a sonicated protein preparation from (i) bacteria harvested from in vivo- and (ii) from in vitro-cultured bacteria, plus formalin-killed bacteria harvested from the (iii) in vitro and (iv) the in vivo environment. The hypothesis was that both types of vaccines (either killed bacteria or protein preparation) prepared from Salmonellae harvested directly from the chicken intestine would be more immuno-protective than those cultured in vitro in nutrient broth, as they were prepared from the same environment where protection would be required (gut). The protective effect of formalin-killed bacteria plus protein preparations was assessed for their effect in chickens against colonisation and systemic invasion of the homologous challenge strain. Caecal colonisation was assessed by cloacal swabbing with a semi-quantitative method of enumeration which has been used extensively for large groups of birds10,11,18 Some authors,19-21 have shown that killed Salmonella vaccines induce just partial immune protection in poultry. Newly hatched chickens have no established gut flora which is why Avigard gut flora was provided on day one to make available a gut flora that is naturally present in adult birds22 and to reduce the level of coloisation to something more closely resembling that of the adult.
After vaccination Cloacal swabbing has demonstrated to be a useful semi-quantitative method for the faecal shedding of Salmonellae and estimation of caecal colonisation when chickens experimentally infected, as found previously.10,11,18 However, when direct Salmonella counts were made from caecal contents and compared at the end of vaccination experiment I (orally challenged), there appeared little correlation with the semi-quantitative measures determined by Cloacal swabbing. This phenomenon is well known, and is probably assoCiated with intermittent caecal evacuation.23 In the present work when birds were challenged orally to assess Salmonella caecal colonisation by Cloacal swabbing (Experiment I), the response was great enough to significantly prevent caecal colonisation completely in the groups of birds challenged orally with a virulent S. Enteritidis NalR strain when the vaccine was the protein preparation harvested from either the in vivo condition (in chickens) or in vitro (in nutrient broth culture). The results show that protection by both in vitro and in vivo proteins preparation were statistically significant (P<0.001) in their ability to protect against Salmonella colonization. However, the level of protective immunity induced by the in vitro protein preparation was higher than that induced by the in vivo preparation.
The good level of protection induced by the in vitro preparation is in agreement with the previous work conducted by Khan et al.,6 who found that outer membrane proteins of Salmonella when inoCulated with adjuvant are effective against S. Enteritidis in chickens.6 In contrast, the protection produced by formalin-killed Salmonella harvested from either in vitro or in vivo conditions when used as vaccine candidate in chickens was found to be statistically insignificant. This is in agreement with other researchers who found that proteins (OMPs) gave better protection than formalin-killed bacteria.25 For other organisms, such as S. Gallinarumand Pasteurella multoCida, OMPs also give better protection than whole bacterins or sonicated cells.25,26 Immunization with Helicobacter whole-cell extracts or purified components also effectively prevented H. pylori infection in mice.27-30 Protein subunit antigens induce production of antibodies and can also induce cell-mediated immunity, resulting in long-lasting protection against bacterial infections.31
At 4th week post infection no Salmonellae were detected from caecal swabs from the vaccinated groups (<1 x 102cfu/ml) compared with unimmunised group that show the percentage of Salmonellae positive to be 35%.However, these results showed that the better protection induced by the proteins from the in vitro cultured bacteria in comparison with the proteins from the in vivo harvest bacteria were unexpected, since it was anticipated that the in vivo preparation would have been at least as immunogenic as the in vitro preparation, as the protein concentrations of both in vivo and in vitro preparation were similar. However, the reason that the effectiveness of the in vivo preparation as vaccine was less than that of the in vitro preparation may have been the result of degradation of the proteins harvested from chicken caeca by avian proteases as discussed earlier (Chapter 4). The in vivo preparation would have contained a number of antigens that are expressed in the very earliest stages of infection and these may have been important. Moreover, certain genes that encode some important antigens such as LPS and flagella were down-regulated in the intestine of chickens.17,32
It is also known that genes assoCiated with invasion and early infection, including Salmonella Pathogenicity Island 1 (SPI1) genes are up-regulated in the intestine and during infection of epithelial cells but are largely down-regulated during infection of macrophage like cells.33,34 In contrast, Jones et al.35 have reported that in chicken S. Typhimurium SPI-1 system is involved in both systemic infection and gut colonization, but does not appear completely crucial for either infection proCess, and in one day old chicks SPI-1 TTSS is not important in the initial stages of infection.35 However, in S. Pullorum SPI-1 contributes to disease in poultry during infection.36 In chickens the majority of S. Typhimurium genes involved in flagella production including flgM, flgN, flgK, flgB, fliC, fljB, fliA are down-regulated.17 These are highly immunogenic and immuno-modulatory proteins and their down-regulation in the intestine suggests that a vaccine prepared from bacteria harvested from the intestine may, in fact, express lower levels of key immunogens. The altered expression of these antigens; play a key functional role in establishing infection.37-39 It is been reported that flagella play a role in the pathogenesis in chickens40,41 and mouse.42,43 The result of this study is in agreement with previous work conducted by Toyota-Hanatani et al.,44 suggesting that a part polypeptide in S. Enteritidis Fli-C (SEp 9) inhibits S. Enteritidis colonization in the intestine of chickens two weeks after challenge, similarly to commercial inactivated S. Enteritidis vaccine,44 S. Enteritidis colonization inhibition by SEp 9 alone may have been comparable to that by commercial inactivated S. Enteritidis vaccine.
In S. Typhimurium the SPI-2 TTSS plays a role in both intestinal colonisation and systemic infection of chickens.35 Furthermore, microarray work conducted within our group demonstrated that genes of S. Enteritidis Pathogenicity Island (SPI-1, SPI-2 and SPI-5) are up-regulated in vivo indicating a close assoCiation with the mucosa during colonization.32 It is thus likely that the antigenic profile of Salmonella during the infection of antigen-presenting cells is very different from that of Salmonellae during intestinal colonisation, or that the proteins may have some immune-suppressive effects.45 The immunogenicity of bacteria harvested from macrophage infections has not been assessed but given that the biology of Salmonella organisms is very different in the gut and in macrophages,17,32,33 as candidate antigen presenting cells, this would be a worthwhile experiment to do. This may suggest that growth of bacteria in the gut to generate a more rational inactivated vaccine may be less important than culturing them in the conditions found within an antigen presenting cell.
As we ensured that the protein concentrations in the vaccine preparations prepared from the in vivo and in vitro cultures were similar and obtained from a similar number of bacteria and we can state that the in vitro bacteria did not produce larger amounts of protein compared to the in vivo bacteria. So, the difference may lie in the levels of specific proteins expressed under the different conditions of culture. Thus, in contrast to expectations, in vivo harvested proteins were poorer immunogens than proteins harvested from Salmonellae grown in nutrient broth but were better than killed-bacterins.
Formalin killed bacteria as vaccines were therefore found to be poorly protective against faecal shedding. This may be as a result of Salmonella antigen damage during formalization or as a consequence of enzymatic degradation of Salmonella in vivo proteins by avian proteases as found in this study. However, the in vitrocultured formalin-killed bacteria had a slightly better effect than the in vivo bacteria but this was statically not significant. However, formalin killed Salmonella bacterins are used commercially in the field to control the losses acquired by Salmonella in calve in many countries.46,47
The caeca empty and fill several times a day and therefore, the phases of growth of the inoCulated bacteria in individual birds may be different and a considerable proportion of the cells may be in the stationary phase. In addition, the fall in temperature during the specific experiment before challenge might explain the poor immunity provided for groups treated with this vaccine. Inactivated vaccines can reduce S. Enteritidis faecal excretion and that effect may depend on their composition.48 Other authors have suggested that several factors may account for this effect, such as adjuvant type and composition, S. Enteritidis strain and inactivation method.49,50
In this study (experiment II), when birds were challenged intravenously (systemic infection), to asses Salmonella systemic invasion of internal organs such as spleen and liver, no bacteria were observed in the caecal swabs collected from all birds. This observation is different from what has been reported previously where S. Enteritidis was shed in faeces after intravenous challenge.24,51,52 The clearance of the challenge strain from internal organs in both vaccinated and unvaccinated birds were similar. During systemic infection following intravenous challenge the macrophage interaction with Salmonellae is the key in the progress of the systemic infection.53 Salmonella clearance into gastrointestinal tract from the tissues is through gall bladder.24 It is been previously reported that in chickens biliary antibodies are involved in S. Typhimurium clearance from the gut.54 This observation correlated with the results of Woodward et al.54 who reported that the Salmonella count in gall bladder is higher in unimmunised group compared with vaccinated birds.55 However, other authors used a similar route of challenge and reported that bacterial shedding in the faces reached the highest number 1 - 2 weeks post infection,52 which might explain the absence of Salmonella from the caecal sample at day 8.
In the present study a combination of inactivated vaccines and CE was used, with all groups of birds in oCulated with Avigard microflora product prior to exposure to the corresponding vaccine. Such a combination might be considered as a control measure to control infection by Salmonella. Experimentally, a combination of live and CE products was shown to provide a considerable degree of protection of chickens against S. Typhimurium.56
Protection of the chicken can oCcur from transfer of passive immunity to broiler and layers, so a killed vaccine might be useful and used under these circumstances. However, secretory IgA responses which are thought to play a central role in mucosal surface protection cannot be elicited by killed Salmonellae vaccine.57,58
Poultry immunisation against Salmonellae is considered as an important contributory measure to infection control. In chickens vaccination may reduce the severity and period of infection and help avoid re-infection,59 which indirectly should reduce the number of human food-borne salmonellosis cases.60 It has been shown that inactivated vaccines of S. Enteritidis can induce a good humoral immune response, and induce some reduction in intestinal colonization by S. Enteritidis.57,61-63
Previous studies indicated that humoral responses are less important than cell-mediated immunity in protection against Salmonellae.64,65 Other published research agreed that cell mediated immunity determines the overall defense against systemic salmonellosis64,66 probably even in the intestine.67 Several studies have reported that live vaccines are more effective and provide greater protection than killed vaccines in protecting birds from S. Enteritidis infection.61,68-70 Much earlier work indicated that compared with a live vaccine killed bacteria do not induce as strong a protective response in controlling fowl typhoid,71 and also disease-free intestinal carriage of Salmonella19,20 as indicated previously.
It is not clear if one of the reasons is antigen destruction during vaccine preparation or whether in a related way persistent presentation of the antigen on active multiplying bacterial cells is necessary for stimulation. Other researchers have tested S. Enteritidis inactivated vaccines (Bacterin with Modified Freund’s adjuvant) and demonstrated a variable reduction in the S. Enteritidis faecal shedding; the best protection was obtained by bacterins with modified Freund’s adjuvant, with the S. Enteritidis cells inactivated by 20% acetone.50 An inactivated S. Enteritidis PT4 which comprised 1011 cfu/ml in 50% oil adjuvant was also developed by Timms et al.24 which provided a significant protection against both intramuscular and intravenous challenge 5 and 8 weeks post challenge when it inoculated intramuscularly24 although it must be remembered that this was parenteral challenge with a bias towards the vaccine. One other reason for reduced immunogenicity of killed Salmonella vaccines is that the presented antigens are expressed under the conditions of culture which in most cases is a rich medium in vitro.72 Conversely, live attenuated vaccines may be antibiotic resistant and may be unsafe due to insufficient attenuation.73,74 The most extensive currently used inactivated vaccine is produced by culturing Salmonella bacteria under conditions of iron restriction, on the basis that this will generate immunogenic surface proteins required for iron uptake.52 However, other factors besides iron restriction are important during infection of macrophages,33 or of the intestine.32,75 Killed vaccines stimulate antibody production and express only the antigens present at the time of in vitro harvesting.64,72 Killed vaccines may be eliminated and destroyed rapidly, with poor immunogenicity in unprimed hosts.63,76 In contrast to crude undefined preparations perhaps the future route is through specified proteins known to be immunogenic such as the outer membrane proteins (OMP). Besides lipids and lip polysaccharides the outer membrane of Gram negative bacteria contain some proteins which represent integral proteins of the bacterial outer membrane.77 Salmonella OMP play a vital role in the stimulation ofimmune response and may be used as effective vaccine candidates providing active immunity. A previous study used S. Enteritidis isolated from chicken meat showed that OMPs of molecular weight 14.4 - 24 KDa are immunogenic and might be used for vaccine preparation to control salmonellosis in human and animals.77 Another study revealed that S. Typhimurium strains contain OMPs of 37 and 40 and 41.7 KDa.78 S. Enteritidis OMPs are effective in reducing colonization of S. Enteritidis on intestinal mucosa in chickens and could be used as potential vaccines to reduce S. Enteritidis colonization in chickens.6 (Adjuvanted OMP vaccines gave better protection than sonicated extract of S. Enteritidis vaccines.79 Although Salmonella bacterins may not be effective against intestinal colonization, albeit moderately against invasion of chicken visceral organs,80 better protection can be obtained in laying hens when a combination of both live S. Enteritidis, followed by a bacteria is used.81 In poultry vaccines against Salmonella infection are thus incompletely effective, and must be seen as a single component in Salmonella control regimens involving a combination of vaccination programs together with hygienic measures.
The authors declare no conflict of interest.
None.
None.

©2017 Elazomi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.