Journal of
eISSN: 2373-437X


Research Article Volume 6 Issue 2
Department of Food and Animal Science, Universitat Autonoma de Barcelona, Spain
Correspondence: Jose J Rodr¡guez Jerez, Department of Food and Animal Science, Faculty of Veterinary Science, Universitat Autonoma de Barcelona, Spain , Tel 34 935811448, Fax 34 933807558
Received: December 23, 2017 | Published: March 9, 2018
Citation: Ripolles-Avila C, Ríos-Castillo AG, Guerrero-Navarro AE, et al. Reinterpretation of a classic method for the quantification of cell density within biofilms of Listeria monocytogenes. J Microbiol Exp. 2018;6(2):70-75. DOI: 10.15406/jmen.2018.06.00190
Food product contamination by Listeria monocytogenes is mainly related to the pathogen’s ability to form biofilms on food processing surfaces. This study aims to correlate a method based on a microplate with a final quantification of the cell density in CFU cm-2 within the biofilms formed by different strains of L. monocytogenes. It is considered that being aware of the capacity that different strains of L. monocytogenes possess to form biofilms is highly relevant to address specific cleaning and disinfection procedures in industrial environments. A total of 17 strains with distinct origins and genetic characteristics were employed in this study. Despite all strains were shown to be capable of forming biofilms, significant differences were observed between strains. The data obtained suggests that working with cell counts over a surface is more accurate than absorbance values when regarding biofilms, and therefore our reinterpretation of the classic assay is a quick and manageable method for classifying biofilm producer strains prior to carrying out more complex analyses.
Keywords: Listeria monocytogenes, biofilms, quantification, biofilms detection, food hygiene
BPW, buffered peptone water; CECT, spanish type culture collection; CFU, colony forming units; INIA, national research institute, agricultural and food technology; OD values, optical density values; TSA, tryptone soya agar; TSB, tryptone soya broth; TSYEB, tryptone soya yeast extract broth
Food safety is one of the biggest concerns of food industrial development.1 This is mainly related to foodborne diseases, which are the most widespread health problem in the contemporary world and an important cause of reduced economic productivity.2 Listeria monocytogenes is an ubiquitous organism naturally present in soil and water, capable of contaminating a wide variety of foods when introduced into food-processing environments due to its hardy growth characteristics.3–5 It causes human listeriosis, an infection with a low incidence but a high mortality rate amongst human populations with vulnerable immune systems and the elderly.6 This, along with its connection with stillbirth and miscarriage, makes it a considerable public health problem.7
L.monocytogenes is capable of forming biofilms on food processing surfaces.8 A biofilm is an aggregation of microorganisms growing on a surface and embedded in a matrix of self-produced extracellular polymeric substances which exhibit an altered phenotype as regards growth rate and gene transcription.9–13 These biofilms can act as reservoirs for spoilage or pathogenic bacteria and are directly related to product contamination.14,15 It has been observed that attachment to surfaces and the subsequent biofilm formation confers resistance on the cells to antimicrobials agents, UV light, desiccation and treatments with sanitising agents, which is not commonly found when the same cell is planktonic.3,16 Furthermore, the capability of foodborne bacteria to outlast the stresses that are habitually encountered in food processing such as refrigerating, disinfecting, acidity and salinity, is enhanced by biofilm formation.10,17,18
Various direct and indirect observation methods have been used to evaluate the biofilm-forming ability of L. monocytogenes.7,9,10,19 Direct methods are based on directly observing the microbial colonisation. These methods include microscopy techniques such as light, transmission electron, laser-scanning confocal, scanning electron and epifluorescence microscopy.8 Blackman und Frank20 observed that not only can biofilm levels be underestimated when epifluorescence microscopy is employed because the thickness is not being measured, but also that the area covered by cells may be overestimated because some extracellular polymer can be stained. Despite this drawback, when studying attached bacteria on surfaces it is important to use direct microscopy to obtain accurate results.21 Indirect methods, on the other hand, are those that detach the microorganisms from the surface before counting them. These methods include not only standard plate counts, roll techniques and sonication, but also other procedures that estimate the number of adhered microorganisms in situ by measuring some feature of the adhered organism.22 These include techniques such as radiolabelled bacteria, enzyme-linked immunosorbent assay, biologic assays, stained bacterial films and microtiter plate procedures.23
Focusing on the microtiter plate procedure, there are some assays related to biofilm formation. One of these is the crystal violet method, which was proposed by Christensen et al.,24 and adapted by others.3,8,25 This assay is comprised of growing microbial biofilms on microtiter plates, staining them with crystal violet and then solubilising the bound dye to measure its concentration. It has been extensively employed for microbial culture screening.7,26 However, the use of this method only allows the classification of the strains from the most to the lowest biofilm producer, but you do not know the exact number of cells forming the biofilm.
For the food industry, it is completely crucial to be able to detect biofilms formed in food-contact surfaces rapidly in order to assess their hygienic state and take associate decisions regarding to cleaning and disinfection procedures. One of the methods that can be employed is the use of biodetectors such as Bio finder that detects the presence of biofilms on surfaces within seconds, allowing the food industry to visually identify if biofilm exist in the food-contact surface. This study aimed to investigate the potential for adapting an existing method that evaluates biofilm production qualitatively to a method that quantifies exactly microbial biomass to polystyrene. The main purpose of this study was to quantify the cell density within biofilms of different strains of L. monocytogenes using crystal violet assay with a further reinterpretation of this classic method to obtain the number of cells forming the biofilm. For the purposes of developing the assay, a correlation curve between Optical Density (OD) values and Colony Forming Units (CFU) per square centimetre was performed. The different L. monocytogenes strains were compared in order to investigate their potential as a biofilm formers. Finally, some of the wells on where the biofilm was formed, were observed by microscopy and others, were examined using a biofilm biodetector.
Characterisation of isolates
Five culture collection strains (CECT) and twelve isolates of L. monocytogenes were used in this study (sources and serotype are listed in Table 1).27 These seventeen different strains were selected for the study as the basis for screening strains that belong to distinct linages. Procedures for collecting, identifying and typifying some of these isolates by serotyping have been previously described.28 The different bacterial strains were stored at 4ºC as frozen-dried cultures, which were then recovered on Buffered Peptone Water (BPW, bioMérieux®, Marcy l’Etoile, France), cultivated at 30ºC for 48 hours, streaked onto Tryptone Soya Agar (TSA) (CM0131, Oxoid, Spain) and cultivated at 30ºC for 48 hours. Finally, working cultures were kept on TSA slants at 4ºC to be used within 30 days.
|
Isolates |
Serovar |
Origin* |
|
4423 |
1/2a |
Ortiz et al.,28 |
|
5366 |
4b |
CECT |
|
5672 |
4b |
CECT |
|
5873 |
1/2a |
CECT |
|
911 |
1/2c |
CECT |
|
935 |
4b |
CECT |
|
A7 |
1/2a |
INIA |
|
CDL69 |
1/2a |
Ortiz et al.,28 |
|
EGD-e |
1/2a |
Ortiz et al.,28 |
|
P12 |
1/2a |
INIA |
|
R6 |
1/2a |
INIA |
|
S1(R) |
1/2a |
INIA |
|
S1(S) |
1/2a |
INIA |
|
S10-1 |
2a |
Ortiz et al.,28 |
|
S2-1 |
1/2a |
INIA |
|
S2-2 |
1/2a |
INIA |
|
S2bac |
1/2a |
Ortiz et al.,27 |
Table 1 Listeria monocytogenes isolates used in this study
*The different isolates were obtained from Spanish Type Culture Collection (CECT) and the National Research Institute, Agricultural and Food Technology (INIA). From Ortiz et al.,28 and Ortiz et al.,27 the isolates were collected during a three-year study in an Iberian pork processing plant.
Spectrophotometric determination of the crystal violet dye
The crystal violet dye solution was prepared at a concentration of 0.8% in sterile distilled water. One millilitre of this solution was dispensed in a spectrophotometric cell. The absorption spectra for the dye solution was measured by the VIS spectrophotometer type Spectroquant® Prove Spectrophotometer 100, Merck Millipore, USA. The wavelength range to establish the absorbance of the crystal violet solution was set from 320 nm to 900 nm.
Biofilm formation on the microtiter plates
Each L. monocytogenes strain was subcultured for 22 hours on TSA and incubated at 37ºC. Biofilm production assays were carried out with a rich undefined medium, TSYEBgluc1%+NaCl2%, which consisted of tryptone soya broth (TSB) (CM0129, Oxoid, Spain) supplemented with 0,3% w/v yeast extract (212750, BD, Spain), 1% w/v glucose (4125012, Biolife, Spain) and 2% w/v sodium chloride (131659, Panreac, Spain). The overnight cultures on TSA were transferred by collecting bacterial colonies from the medium surface to 10 ml of TSYEBgluc1%+NaCl2%, resulting in a suspension of 106CFU ml-1, which was then vortexed. This concentration was standardized by photometric readings with a Densimat photometer (bioMérieux®, Marcy l’Etoile, France). After vortexing, sixteen volumes per strain of 100µl were transferred into PVC microtiter plate (Sudelab, Spain) wells. The microtiter plates had been previously rinsed in 70% ethanol and air dried. The microtiter plates were incubated at 30ºC for 48 hours. Each experiment included sixteen wells of TSYEBgluc1%+NaCl2% medium without L. monocytogenes as control wells.
Biofilm quantification using crystal violet assay
After 48 hours of incubation, medium from each of the wells was withdrawn into a discard container and the microtiter plate wells were washed twice with 100µl of sterile water to eliminate the unattached cells. The plates were then air dried for 45 minutes. Each well was stained by adding 100µl of 0.8 % crystal violet solution in water and the microtiter plates were incubated for 45 minutes at room temperature. After staining, the unbound dye was eliminated and the biofilms were visible as purple rings formed at the base of the well (Figure 1). In order to proceed with the quantitative analysis of the biofilm, the crystal violet was solubilised by adding 150µl of 95% ethanol to fade the wells and was incubated for 30 minutes at room temperature. 100µl from each of these wells was transferred to a new sterile polystyrene microtiter plate and the crystal violet level present in the destaining solution was measured by a microtiter plate reader (340 ATTC, SLT Labinstruments, Vienna - Austria) at an optical density of 595 (OD595). A procedure was performed to correlate the level of crystal violet dye with the number of bacteria stained in the wells. The results were reported as CFU cm-2. The biofilm formation on the polystyrene plates and its subsequent quantification was carried out three times on three different days for all the strains of L. monocytogenes. The averages and standard deviations were calculated for all repetitions of the experiment.
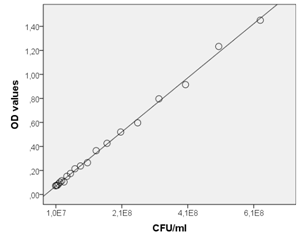
Figure 1 Correlation between the absorbance of crystal violet in OD values and the colony-counting method. Each value corresponds to the mean of three replicates.
Correlation curve between OD values and CFU ml-1
The correlation curve was obtained for the strain of L. monocytogenes CECT 935, which is an international reference of ISO 1129029 and for three more strains L. monocytogenes A7, EDG-e, CECT 5366, which were randomly chosen in order to investigate if there were differences between strains. To create the correlation curve, the overnight cultures on TSA were transferred by looping bacterial colonies to 10 ml of TSYEBgluc1%+NaCl2%, resulting in a suspension of 109CFU ml-1. This was vortexed and one millilitre was then transferred to an Eppendorf tube (Eppendorf, Hamburg, Germany). The samples were centrifuged by the Heraeus Pico 17 Centrifuge, Thermo Scientific for 30 seconds at 12000 rcf. In the inoculated Eppendorf tubes, L. monocytogenes was visible as a bacterial pellet. The supernatant was discharged and the bacterial pellets were washed twice with sterile water. Each Eppendorf tube was stained by adding 1 ml of 0.8% crystal violet solution and incubated for 45 minutes at room temperature. After staining, the unbound dye was eliminated and the pellets were stained purple. The crystal violet was solubilised by adding 1 ml of 95% ethanol, which destained the pellets, and was then incubated for 30 minutes. Serial dilutions of this solution with ethanol were prepared to observe absorbance from high to low (Figure 2). 100µl was transferred from each Eppendorf tube to a polystyrene microtiter plate and the crystal violet level was measured in a microtiter plate reader at an optical density of 595 nm. The optical density values were correlated with the CFU ml-1 obtained by plating on TSA from the initial bacterial suspension.
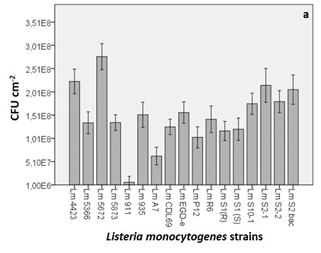
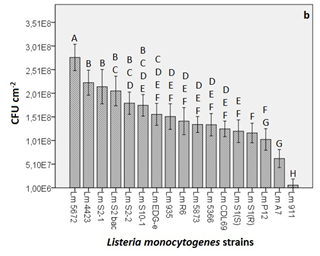
Figure 2 (A) Microbial counts obtained from the quantification of the biofilm formation of different strains of L. monocytogenes (Lm). In the graphs, the y-axis shows the CFU cm-2 and x-axis shows the different strains employed in the study. Each value corresponds to the mean of three replicates. The bars represent the standard error of the mean (B) Strains sorted in descending order from the most major biofilm-producing strain to the least. The graph shows the eight different groups obtained through the Tukey’s B test where significance was set at p<0.05.
Detection of biofilms formed on microtiter plates using a biofilm detector (Biofinder)
The biofilms were formed as described in section 2.3. After a 48-hour incubation period, medium from each of the wells was withdrawn into a discard container and the microtiter plate wells were washed twice with 100µl of sterile water to eliminate the unattached cells. One drop of Biofinder (iTram Higiene, Vic, Spain) was then introduced into the wells containing the 17 different strains of L. monocytogenes. The reaction was observed after 10 seconds.
Microscopic observation of the biofilm formation
L. monocytogenes CECT 935 was the only strain used for this evaluation. The biofilms were formed in the same microtiter plates and stained using the method described in section 2.4. After the biofilms were stained, the wells of the microtiter plate were sliced for observation by microscopy. The main objective was to qualitatively compare the biofilms formed at 48 hours with the biofilms formed after 7 days of incubation. To form the one week incubated biofilms, volumes of 100µl of L. monocytogenes CECT 935 inoculum were transferred into sixteen PVC microtiter plates and incubated for seven days at 30ºC with different washes and renewal of the culture medium at 48-72-24-24 hours of incubation in order to remove the unattached cells and promote the growth of the adhered cells. The washes and renewal of nutrients was done by washing three times with sterile distilled water and 100µl of new sterile TSYEBgluc1%+NaCl2% medium was dispensed into the previously incubated wells.
Statistical analysis and reproducibility of results
Sixteen replicates for each of the three independent repeats were carried out for each trial. The data presented are the means of the data generated from the three independent experiments. The data from all the experiments were analysed by means of the SPSS statistical package using the ANOVA general linear model with subsequent analysis of variance and Tukey’s B test. A p value of <0.05 was considered to be significant.
Spectrum of crystal violet dye
The absorption spectrum of the 0.8% crystal violet solution was performed not only to investigate where the maximum absorption peak was, but also to see if it concurred with the previously published literature. The maximum absorption peak was observed at 595 nm and therefore this was the wavelength employed in the study. The results were consistent with the published literature on where preliminary studies using microtiter plates for assessing L. monocytogenes biofilm formation had used the same wavelength.3,8,30
Growth of L. monocytogenes on TSYEBgluc1%+NaCl2%
Seventeen different L. monocytogenes strains were tested using a modified crystal violet assay for their quantification. The evaluation of their biofilm-forming ability was carried out after a 48-hour incubation period at 30ºC. The media employed was the one proposed by Pan et al.,31 which was TSYEB with a supplement of glucose and sodium chloride.
L. monocytogenes is an ubiquitous organism that can grow in a large variety of environments using different nutrient sources.32 Preceding studies have shown that the growth and subsequent biofilm formation of L. monocytogenes is affected by the growth media used.33 Pan et al.,31 demonstrated that sodium chloride and glucose concentrations play a crucial role in the formation of biofilms. In his study, TSYEBgluc1%+NaCl2% medium was employed as a growth media and almost all the L. monocytogenes strains exhibited enhanced biofilm formation at 30ºC when 2% salt and 1% glucose concentrations were added. Hence, TSYEB with glucose and sodium chloride was chosen for the study. For developing the assay with optimum conditions, apart from the growth medium, growth temperature as an influential parameter for the biofilm formation was also assessed. The formation of biofilms was much lower at temperatures of 12ºC and 22ºC than at 30ºC (data not shown), so 30ºC was the temperature selected for this research study.
Correlation curve for the biofilm quantification
The method was initially proposed by Christensen et al.,24 and adapted by other authors.3,8,25 With the reinterpretation suggested in this study, however, the results will be obtained as microbial counts which reflects more accurately the biomass of the formed biofilms. To achieve this, a correlation between OD595 values and CFU ml-1 was performed to set up a correlation curve as a basis for comparing the OD595 values from the experiments with estimates of the numbers of bacteria represented by these OD595 values, as other authors have done.34 The measured data on the relationship between OD values and CFU ml-1 for L. monocytogenes CECT 935 is shown in Figure 1. The results indicate that the method is enormously reliable for quantifying L. monocytogenes organisms. A curve representing the relationship between OD values and the corresponding cells counts was plotted and analysed using linear regression. The bacterial cells showed a linear relationship in the range of 1x107–x109 cells ml-1 and the correlation between the two methods was high (coefficient of determination, R2=0.998). Any statistically differences were found between the correlation curves performed using the four strains of L. monocytogenes and therefore it was proved that the same correlation curve can be used to estimate the number of the cells forming the biofilms of the seventeen different strains of L. monocytogenes. In addition, obtaining the results as microbial counts is a better and more useful tool for simultaneously comparing different quantification methods.
Quantification of the biofilm formation on polystyrene microtiter plates
The biofilm formation was investigated statically after a 48-hour incubation period. Under these conditions, the OD595 values of crystal violet obtained from the faded biofilm varied greatly for the different strains of L. monocytogenes. Using the standard curve shown in Figure 1 and correcting the value to obtain cm2, CFU cm-2 readings ranging from 108 to 106 were observed (Figure 2A).
When the Tukey’s B test was applied, 8 different groups (codified from A to H) with significant differences (p<0.05) between them were found (Figure 2B). In those groups, there were strains that belonged to a single group (e.g. Lm 5672, 4423, S2-1, S1(R), A7 and 911) and others that could be classified into more than just one group. For example, strain Lm 5672 fits into group A only, whilst strain Lm S2bac fit into groups B and C.
All of the different strains of L. monocytogenes were found to be capable of forming biofilms, although some of the strains were considered to be better biofilm formers on the basis of the difference in the microbial counts (e.g. 2 log difference between the strongest and the weakest biofilm producer strain). L. monocytogenes CECT 5672, categorised in group A, was the strongest biofilm producer and was significantly different (p<0.05) from the rest of the strains. L. monocytogenes CECT 911, categorised in group H, was the weakest biofilm producer with the minimum cell count level detected and significant differences (p<0.05) from the rest of the strains were also observed. L. monocytogenes 4423, S2-1, S2bac, S2-2 and S10-1 were categorised in group B, meaning that no significant differences (p=0.298) were observed among them. With the rest of the strains, however, significant differences (p<0.05) were found, except for L. monocytogenes S2bac, S2-2 and S10-1, which were categorised in more than one group. For example, in the case of L. monocytogenes S2bac, not only were no significant differences observed among the strains that belonged to group B, but no significant differences (p=0.06) were noticed with L. monocytogenes S2-2, S10-1 and EDG-e either (group C). The most similar strains regarding their capacity to produce biofilms were thought to be the following: L. monocytogenes EDG-e, R6, CDL69 and CECT 935, 5873, 5366 which were categorised simultaneously in three groups D, E and F. These strains presented less significant differences when compared with the rest of strains, thus they were considered to share more similarities.
The seventeen strains employed in this study belonged to different serotypes (see Table 1 in chapter 2.1), which can be grouped into divisions. Division I consists of serotype 4b, Division II consists of serotypes 1/2a and 1/2c and strains belonging to serotypes 2a are considered as other serotypes. Variations in biofilm production at the level of divisions was also observed, but there were no significant differences. In contrast, other studies have found statistically significant differences when correlating the formation of biofilms with phylogenetic division.3,8 This can be ascribed to the material employed as varying adhesion between different lots of microtiter plates has been reported.22
Detection of biofilms formed on the microtiter plates using Biofinder
Biofilm formation detection of the different strains on polystyrene surfaces was also assayed using the product Biofinder. The results (Figure 3) showed not only a strong positive reaction with plentiful bubble formation in the different wells, but they also demonstrated that the intensity of the bubbles was higher in the strains that were found to be more biofilm-producing than the ones that were less biofilm-producing. The present study demonstrated prolific bubble formation, which was considered to be an intense and positive reaction. Moreover, the use of this product also allowed us to screen the bacterial strains from the strongest biofilm producer to the weakest. The results concurred with those obtained when quantifying the biofilms formed with the different strains of L. monocytogenes, whereas this product can also be used as a screening tool to identify which strains are stronger and weaker biofilm producers.

Figure 3 Application of the biodetector to the seventeen strains after biofilm formation. It includes a control well for observing negative reaction.
Microscopic observation of crystal violet stained biofilms
A qualitative study was conducted to observe not only the typical morphology of the biofilms, but also to show how they increase over the time (Figure 4). The percentage of covered areas at 48 hours of incubation was lower than the percentage after one week of incubation. Figure 4B shows compact biofilm agglomerations with high cell density, as opposed to Figure 4A, which presents a more distributed network of microcolonies all over the polystyrene surface. This is important for visually determining the optimal biofilm network and for further protocols to develop more agglomerated biofilms. For further research, biofilms should be developed under an incubation period of one week due to they showed more compact agglomeration and a higher cell density, which can helps us to better understand biofilm behaviour in its mature stage.
Crystal violet assay is a rapid and effective method to analyse bacterial adhesion and it allows different parameters to be tested within the same experiment. For the researcher this means a quick, previous step for screening and evaluating different test conditions. Based on the results obtained and the literature survey, it can be concluded that the reinterpretation of this classic method is useful due to it makes quantification as microbial counts possible, it makes comparison with other methods to be easier and it gives more accurate results. Further research is needed to explore the use of this method to rapidly quantify biofilm production on other materials. Extrapolating and adapting the technique to assess the biofilm-forming ability of different bacterial strains could be immediately advantageous in that it means finally having less labour-intensive quantification techniques.
The authors thank Dolors Busquets enormously for her technical assistance. This study was supported by Research Project grants RTA2014-00045-C03-03 (INIA) from the Spanish Ministry of Economy and Competitiveness. The support of the company Itram Higiene (Vic, Barcelona, Spain) is also gratefully acknowledged.
The authors thank Dolors Busquets enormously for her technical assistance. This study was supported by Research Project grants RTA2014-00045-C03-03 (INIA) from the Spanish Ministry of Economy and Competitiveness. The support of the company Itram Higiene (Vic, Barcelona, Spain) is also gratefully acknowledged.

©2018 Ripolles-Avila, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.