Journal of
eISSN: 2373-437X


Review Article Volume 9 Issue 4
1Estudiante Microbiología y Bioanálisis, Grupo de investigación Microbiología Ambiental, Escuela de Microbiología, Universidad de Antioquia, Colombia
2Bact, MSc Ciencias Básicas Biomédicas, PhD Microbiología y parasitología, Universidad de Antioquia, Colombia
3MyB, MSc Epidemiología, MSc Economía aplicada, PhD (c) Salud Pública, Escuela de Microbiología Universidad de Antioquia, Colombia
Correspondence:
Received: June 16, 2021 | Published: July 15, 2021
Citation: Agudelo SG, Diaz ALG, Cardona-Arias JA. Prevalence of Plasmodium spp. and helminths: Systematic review 2000-2018. J Microbiol Exp. 2021;9(4):107-119. DOI: 10.15406/jmen.2021.09.00331
Introduction: The parasites that cause malaria and helminthiases are distributed in the same geographical areas, affect the same groups and share risk factors; however, its coinfection is little studied.
Objective: To estimate the global and specific prevalence by species of Plasmodium spp., Helminths and their coinfection based on studies published in the world scientific literature, 2000-2018.
Methodology: Systematic review of the scientific literature based on studies published in Pubmed, Science Direct, SciELO, Web of Science, EBSCO and Google Scholar. Investigations were included based on the implementation of a search protocol that included inclusion and exclusion criteria, according to the PRISMA guide. Reproducibility of the search and selection of studies was guaranteed. The methodological quality was evaluated with STROBE.
Results: We included 61 articles with a population of 45,060 people, mostly from Africa, with children and pregnant women. 51 evaluated coinfection in the general population and 10 analyzed helminth infection in a population with malaria. The prevalence of malaria was 41%, helminths 43.4% and the coinfection 17.2%. The most prevalent species were Plasmodium falciparum, Schistosoma haematobium, Uncinarias and Ascaris Lumbricoides. The coinfection between Plasmodium falciparum and Uncinarias was the most prevalent with 6.1% in the general population and 28% in people with malaria.
Conclusion: We found a high prevalence of coinfection in a small number of studies, which shows that the study of the interactions between Plasmodium and helminths is an undeveloped area in parasitology. Despite the high magnitude of malaria and helminths in the Americas, studies of coinfection in the region are scarce, which constitutes an obstacle to impact its clinical and epidemiological effects, while preventing the development of public policies for parasitological control in endemic areas.
Keywords: malaria, helminths, coinfection, prevalence, Plasmodium spp, geohelminths
Malaria is a disease transmitted to humans by the bite of infected female mosquitoes, of the genus Anopheles, it is caused by parasites of the genus Plasmodium spp. and the species that infect humans are Plasmodium falciparum; Plasmodium vivax; Plasmodium malariae; Plasmodium ovale. Plasmodium knowlesi and recently Plasmodium cynomolgi, the first two being the most prevalent.1 The symptoms of malaria occur due to the invasion of the parasites into the erythrocytes where they reproduce and cause their lysis, generating tissue anoxia, immune mechanisms such as fever and immune complexes, bleeding disorders and anemia also occur.2
According to the World Malaria Report, there were 216 million cases of malaria in 2016, compared with 211 million in 20151 and the estimated number of deaths from malaria was 445,000, similar to that of 2015 (446,000). The African continent recorded 90% of the cases and 91% of the deaths from this cause, and 15 countries in the sub-Saharan region reported 80% of the burden of the disease.3 The living conditions of people who inhabit malaria endemic places converge with areas of high risk or susceptibility to contracting helminth infection, for example, the storage of water in tanks or wells can be contaminated with the infectious forms of some helminths as well that favor the reproduction of the malaria vector. To this are added other links of epidemiological and clinical type, by sharing some environmental, sociodemographic and economic risk factors; affect the same type of subgroups and cause effects that can aggravate the clinical condition of susceptible populations such as children and pregnant women.3
In this sense, helminthiases are one of the most common parasites in the world and affect the poorest and most disadvantaged communities. They are acquired by the consumption of eggs, the penetration of larvae as in the case of hookworms and schistosomiasis, the consumption of undercooked meat with the larval phase of cestodes or the bite of mosquitoes in the case of filariae.4 In the world, approximately 1.5 billion people, almost 21% of the population, are infected by soil-transmitted helminths; 200 million people suffer from schistosomiasis and 120 million affected by filariasis.4 Helminthiases are widely distributed throughout the tropics and subtropics, especially in sub-Saharan Africa, Central America, South America, and East Asia.
More than 267 million preschool-age children and more than 568 million school-age children live in areas with intense transmission of these parasites and need treatment and preventive interventions.5 Helminthiasis corresponds to a public health problem, especially in developing countries, where poverty, malnutrition, inadequate water sanitation and minimal health care prevail. The highest rates of infection are usually found in children 5 to 15 years of age and women of childbearing age.6 People with helminth infections present variable symptoms such as transient pruritic lesions on the skin (due to the penetration of hookworm larvae); digestive disorders such as diarrhea (intestinal helminths), foreign body sensation and anal itching, inflammation of the lymphatic tissue with adenopathy and lymphagitis (in the case of filarial infection); hematuria and dysuria (Schistosoma haematobium infection)2 but the most serious signs and symptoms are anemia, neurological and systemic damage.
In this vein, the complementarity with Plasmodium infection is evident. Thus, malaria is predominantly found in rural areas of tropical zones, especially those with nearby freshwater sources and whose income depends on agricultural production.7 Agricultural practices such as irrigation create favorable conditions for mosquitoes, increasing human exposure to vector-borne diseases;7 they also favor the life cycle of many species of helminths by depositing their eggs and larvae in water sources, which simultaneously function as a breeding ground for mosquitoes of the genus Anopheles spp.8
This indicates that the risk factors for the development of the disease in some cases may be similar for both infections. The combination of both groups of parasites can deteriorate the individual's health status, especially due to the development of severe anemia and malnutrition.7,9 Additionally, the identification of coinfection cases allows health agencies to plan mitigation actions and implement strategies that eliminate the risks of suffering from both diseases.10
In addition to the epidemiological and clinical links exposed, in research terms, various studies have shown that communities living in tropical regions of low-income countries have a higher burden of malaria infection; a high prevalence of helminth infections has been documented in these same áreas.10 The Plasmodium falciparum infections can occur in association with intestinal helminths such as Ascaris lumbricoides, Trichuris trichura and Hookworms, association with Schistosoma haematobium and Schistosoma mansoni can also be found in endemic areas for these helminths.10 This coinfection between helminths and malaria has been described as a result of similar life cycles and modes of infection, as well as adequate environmental factors and human practices that favor the circulation of these parasites,11 to which is added the research of their Deleterious physiological links as the cause of nutrient and blood loss.12
Despite the above, studies in this field of parasitology are scarce and to date there is no study that groups and summarizes the available evidence, which is of great relevance given that coinfection constitutes a public health problem that is little addressed, and clinically, the effects of polyparasitism on human health are poorly understood, for which an observational study is required to show the magnitude of the problem and the main causal agents. Therefore, the objective of this research was to estimate the global and species-specific prevalence of Plasmodium spp, helminths and their co-infection from studies published in the world scientific literature between 2000 and 2018.
Type of study: Systematic review of the literature.
Study search and selection protocol: The identification, screening, selection and inclusion phases of the PRISMA guide (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) were applied.
Identification: The search terms from the DeCS thesaurus, “Helminthiasis”, “Malaria” and “Helminths” were used and also combinatorial with the following terms were included: Plasmodium Vivax; Plasmodium falciparum; Plasmodium malariae; Plasmodium knowlesi; Plasmodium ovale and Plasmodium cynolmogi.
The search was carried out in Pubmed, Science Direct, SciELO, Web of Science, EBSCO/Medline and Google Scholar, using the Boolean AND. Some syntax used were: in SciELO (ti (Helminths AND Malaria)); in Pubmed (Helminthiasis [TitleAbstract]) AND Malaria [TitleAbstract]; in Science Direct: Title, abstract, keywords Helminths Malaria; in Web of Science Title, abstract, keywords Helminthiasis Malaria, in EBSCO TI Helminthiasis AND TI Malaria and in Google Scholar allintitle: Helminthiasis Malaria.
Screening: Based on reading the titles and abstracts of the manuscripts, the following inclusion criteria were applied: a) having the search terms in the title or abstract (in the case of Google Scholar, only the title filter applies), b) publications of research in humans, c) be an original study, and d) whose objective was the prevalence of coinfection between helminths and malaria. No time restrictions were applied retrospectively, the search ended on August 1, 2018.
Choice: Those that met the following exclusion criteria were eliminated: those that were not available, that dealt with a topic other than helminthiasis and malaria, that were prevalence studies without coinfection data, and those in a language other than English, Spanish and Portuguese.
Inclusion: Studies that completed the previous phases were included, the following variables were organized and extracted: title; Author; year; country; the purpose of the study; population (n); description of the population; study groups; sub groups; population with helminths, population with malaria and population with helminth/malaria coinfection; helminth species and prevalence; Plasmodium spp species and prevalence; prevalence of coinfection and species involved. Diagnostic method for malaria; diagnostic method for helminths.
Reproducibility and evaluation of the methodological quality of the studies
In this review, the reproducibility in the search and the selection of the publications was guaranteed by applying the protocol on two different occasions, with an interval of one week, while an Excel database was designed for the reproducibility of the information extraction. Which was completed on two different occasions to verify the concordance of the extracted data. A priori it was determined that the discrepancies would be resolved by consensus. To assess the quality of the publications, the 22 criteria of the STROBE guide (Strengthening the Reporting of OBservational studies in Epidemiology) were applied, calculating the percentage of studies that met each of the guide's ítems.
Analysis: A qualitative synthesis was carried out of the predefined variables in the research protocol. Subsequently, the percentage of studies that included data from coinfection between helminths and malaria. The number of helminth-infected subjects infected with Plasmodium spp. and the number of subjects who have coinfection, these data were organized in Excel Office 365 software and analyzed with IBM SPSS Statistics 25.0® software.
193,182 publications were identified, of which 1,244 included the search terms in the title or abstract, which were reduced to 61 when applying the inclusion and exclusion criteria (Figure 1). The articles presented good methodological quality, meeting more than 15 (70%) of the 22 criteria of the STROBE guideline; However, in some items a low proportion of studies were found that applied them appropriately, such as the calculation of the sample size and the discussion about the limitations of the generalization of results (Figure 2).
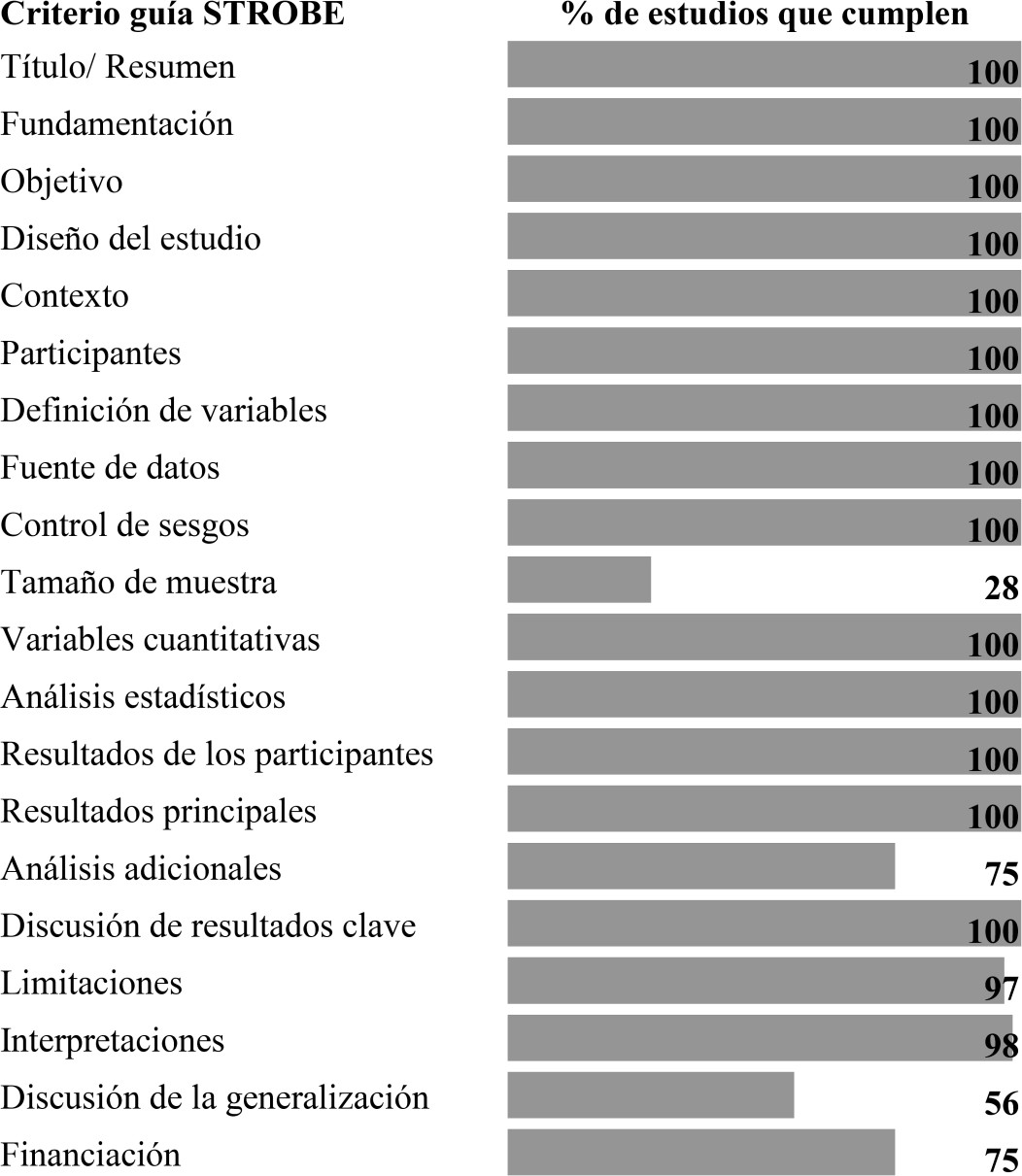
Figure 2 Assessment of methodological quality. Proportion of studies that meet each of the items in the STROBE guide.
The 61 articles were made between 2000 and 2018, with 45,060 people, mainly from Thailand (n = 10 articles), Nigeria (n = 8) and Ethiopia (n = 8); Most of the studies are from the African continent and in America only two studies were found, one from Brazil and the other from Colombia. The populations studied were children (52%), adults (36%) and pregnant women (18%), it should be clarified that 7% of the articles included children and adults. For the analysis, two groups were taken, 51 articles that determined the prevalences of malaria, helminths and its coinfection from the general population and 10 whose population was characterized by having malaria (Table 1).
|
Study |
Year |
Country |
Population |
N |
|
General population |
||||
|
Nacher35 |
2001 |
Tailandia |
Adults |
307 |
|
Egwunyenga36 |
2001 |
Nigeria |
Pregnant |
2.104 |
|
Nacher37 |
2002 |
Tailandia |
Adults |
731 |
|
Le Hesran38 |
2003 |
Senegal |
Kids |
105 |
|
Shapiro39 |
2004 |
Uganda |
Adults |
1.712 |
|
Nkuo40 |
2006 |
Camerún |
Kids |
425 |
|
Bejon41 |
2008 |
Kenia |
Kids |
405 |
|
Midzi42 |
2008 |
Zimbabue |
Kids |
1.303 |
|
Fuseini43 |
2009 |
Ghana |
Pregnant |
300 |
|
Yatich44 |
2009 |
Ghana |
Pregnant |
746 |
|
Yatich45 |
2010 |
Ghana |
Pregnant |
785 |
|
Melo46 |
2010 |
Brasil |
Kids |
216 |
|
Sangweme47 |
2010 |
Zimbabue |
Kids |
605 |
|
Boel48 |
2010 |
Tailandia |
Pregnant |
829 |
|
Yatich34 |
2010 |
Ghana |
Pregnant |
746 |
|
Midzi24 |
2010 |
Zimbabue |
Kids |
609 |
|
Degarege22 |
2010 |
Etiopia |
Kids and Adults |
1.802 |
|
Ojurongbe49 |
2011 |
Nigeria |
Kids |
117 |
|
Idindili50 |
2011 |
Tanzania |
Adults |
464 |
|
Mboera51 |
2011 |
Tanzania |
Kids |
587 |
|
Thigpen52 |
2011 |
Malaui |
Pregnant |
1.772 |
|
Fernández53 |
2012 |
Colombia |
Kids and Adults |
245 |
|
Alemu21 |
2012 |
Etiopia |
Adults |
384 |
|
Ivan54 |
2012 |
Ruanda |
Pregnant |
328 |
|
Righetti32 |
2012 |
Costa de Marfil |
Kids |
324 |
|
Degarege55 |
2012 |
Etiopia |
Adults |
1.065 |
|
Kimbi31 |
2012 |
Camerún |
Kids |
443 |
|
Mulu56 |
2013 |
Etiopia |
Kids |
463 |
|
Ngetich57 |
2013 |
Kenia |
Kids |
356 |
|
Getachew58 |
2013 |
Etiopia |
Pregnant |
388 |
|
Abanye59 |
2013 |
Nigeria |
Niños |
690 |
|
Fairley60 |
2013 |
Kenia |
Pregnant |
696 |
|
Yapi61 |
2013 |
Costa de Marfil |
Kids |
5.246 |
|
Mulu62 |
2014 |
Etiopia |
Adults |
413 |
|
Matangila63 |
2014 |
El Congo |
Kids |
650 |
|
Degarege64 |
2014 |
Etiopia |
Adults |
702 |
|
Kinung'hi65 |
2014 |
Tanzania |
Kids |
1.546 |
|
Ateba66 |
2015 |
Gabón |
Kids |
125 |
|
Ajayi67 |
2015 |
Nigeria |
Kids |
370 |
|
Adedoja68 |
2015 |
Nigeria |
Kids |
1.017 |
|
Salim69 |
2015 |
Tanzania |
Kids |
992 |
|
Ndamukong70 |
2015 |
Camerún |
Kids |
1.138 |
|
Kepha71 |
2015 |
Kenia |
Kids |
5.471 |
|
Njua72 |
2016 |
Camerún |
Kids |
357 |
|
Ateba73 |
2016 |
Gabón |
Kids |
287 |
|
Babamale74 |
2016 |
Nigeria |
Pregnant |
300 |
|
Marcelline75 |
2016 |
Ruanda |
Kids |
465 |
|
Sumbele76 |
2017 |
Camerún |
Kids and Adults |
450 |
|
Adu77 |
2018 |
Ghana |
Adults |
1.569 |
|
Babamale78 |
2018 |
Nigeria |
Kids and Adults |
471 |
|
Bwanika27 |
2018 |
Uganda |
Kids |
240 |
|
Malaria population |
||||
|
Nacher79 |
2000 |
Tailandia |
Adults |
427 |
|
Nacher80 |
2001 |
Tailandia |
Adults |
98 |
|
Nacher81 |
2001 |
Tailandia |
Adults |
179 |
|
Nacher82 |
2001 |
Tailandia |
Adults |
291 |
|
Nacher83 |
2002 |
Tailandia |
Adults |
284 |
|
Nacher84 |
2004 |
Tailandia |
Adults |
438 |
|
Degarege85 |
2009 |
Etiopia |
Adults |
458 |
|
Akanni86 |
2014 |
Nigeria |
Kids |
292 |
|
Othman87 |
2014 |
Malasia |
Adults |
94 |
|
Abbate88 |
2017 |
Tailandia |
Adults |
283 |
Table 1 Description of the included studies according to year, country and population
In the general population the techniques used for the diagnosis of malaria were thick film, PCR and immunochromatography for P. falciparum, with prevalences of 39.9%, 65.9% and 36.3%, respectively, being P. falciparum the species More frequently. The global prevalence of helminths was 43.4%, using Kato-katz, and the sedimentation technique with formalin-ether for the diagnosis of intestinal helminths, urinary sediment for S. haematobium and for filarias, immunochromatography was used, with prevalences of 34.7 %, 29.3% 30.1% and 18.1%, respectively. S. haematobium was the most prevalent agent with 19.9%, followed by filaria with 15.8%. Hookworms were the predominant intestinal helminths (15.5%) followed by Áscaris Lumbricoides with 13.3% (Table 2).
|
|
Evaluated |
Positive |
Prevalence % |
|
Malaria |
|||
|
General frequency |
41.758 |
17.11 |
41,0 |
|
By diagnostic technique |
|||
|
Thick drop |
39.57 |
15.779 |
39,9 |
|
PCR for Malaria |
983 |
648 |
65,9 |
|
Immunochromatography P. falciparum |
1.492 |
542 |
36,3 |
|
By species |
|||
|
Plasmodium falciparum |
34.61 |
12.605 |
36,4 |
|
Plasmodium spp. |
8.5 |
2.571 |
30,2 |
|
Plasmodium vivax |
5.274 |
610 |
11,6 |
|
Plasmodium malariae |
7.858 |
254 |
3,2 |
|
P. falciparum + P. vivax |
3.59 |
52 |
1,4 |
|
Plasmodium Ovale |
5.104 |
16 |
0,3 |
|
Helmintos |
|||
|
General frequency |
41.758 |
18.115 |
43,4 |
|
By Technique |
|||
|
Kato-Katz |
28.117 |
9.77 |
34,7 |
|
Sedimentation with formalin-ether |
8.146 |
2.385 |
29,3 |
|
Urinary sedimentation |
9.742 |
2.931 |
30,1 |
|
Immunochromatography for filariae |
587 |
106 |
18,1 |
|
By species |
|||
|
S. haematobium |
16.049 |
3.196 |
19,9 |
|
W. bancrofti; Very much Mansonella perstans. |
2.275 |
360 |
15,8 |
|
Uncinarias |
36.712 |
5.704 |
15,5 |
|
Ascaris Lumbricoides |
33.4 |
4.452 |
13,3 |
|
Schistosoma Mansoni |
20.55 |
1.79 |
8,7 |
|
Trichuris Trichiura |
32.34 |
2.262 |
7,0 |
|
Strongyloides stercoralis |
6.239 |
253 |
4,1 |
|
Hymenolepis spp. |
5.462 |
207 |
3,8 |
|
Enterobius Vermicularis |
7.466 |
157 |
2,1 |
|
Taenia spp. |
4.404 |
33 |
0,7 |
|
Dicrocoelium spp. |
1.569 |
11 |
0,7 |
|
Trichostrongylus spp. |
1.986 |
7 |
0,4 |
|
Diphyllobothrium latum |
117 |
1 |
0,9 |
Table 2 Prevalence of malaria and helminths in the general population
Seven individuals infected by Trichostrongylus spp. considered a helminth that infects mainly animals, in this case it can be considered a zoonosis; 11 had infection with Dicrocoelium spp. a trematode that mainly infects cattle but humans can act as an accidental host, and a person infected with Diphyllobothrium latum, a cestode whose life cycle includes fish and humans are infected by eating undercooked meat or raw (Table 3).
|
|
Evaluated |
Positive |
Prevalence % |
|
Coinfection Helmintos/Malaria |
41.758 |
7.176 |
17,2 |
|
Plasmodium spp (P. spp.) |
|
||
|
P. spp. + A. lumbricoides |
6.475 |
682 |
10,5 |
|
P. spp. + Uncinarias |
7.134 |
322 |
4,5 |
|
P. spp. + T. trichiura |
6.313 |
312 |
4,9 |
|
P. spp. + S. mansoni |
9.79 |
132 |
1,3 |
|
P. spp. + Hymenolepis spp. |
4.061 |
49 |
1,2 |
|
P. spp. + E. vermicularis |
3.484 |
45 |
1,3 |
|
P. spp. + S. stercolaris |
746 |
21 |
2,8 |
|
P. spp. + Taenia spp. |
3.673 |
18 |
0,5 |
|
P. spp. + S. haematobium |
992 |
2 |
0,2 |
|
Plasmodium falciparum (P.f) |
|
||
|
P.f. + W. bancrofti o Loa loa |
587 |
64 |
10,9 |
|
P.f. + A. lumbricoides |
13.238 |
978 |
7,4 |
|
P.f. + S. haematobium |
12.183 |
845 |
6,9 |
|
P.f. + Uncinarias |
22.166 |
1.343 |
6,1 |
|
P.f. + S. mansoni |
9.79 |
424 |
4,3 |
|
P.f. + T. Trichiura |
11.111 |
451 |
4,1 |
|
P.f. + Hymenolepis spp. |
1.017 |
41 |
4,0 |
|
P.f. + S. stercolaris |
746 |
14 |
1,9 |
|
P.f. + D. latum |
117 |
1 |
0,9 |
|
Poliparasitismo- P. falciparum + |
|
||
|
P.f. + A. Lumbricoides + T. Trichiura |
868 |
57 |
6,6 |
|
P.f. + S. haematobium + S.mansoni+ A. lumbricoides + T. trichiura + Anquilostoma |
2.849 |
45 |
1,6 |
|
P.f. + S. mansoni + Uncinarias |
1.902 |
28 |
1,5 |
|
P.f. + S. haematobium + Uncinarias |
2.133 |
24 |
1,1 |
|
P.f. + S. haematobium +W. bancrofti |
587 |
17 |
2,9 |
|
P.f. + A. lumbricoides + Uncinarias |
1.168 |
16 |
1,4 |
|
P.f. + A. lumbricoides + T. trichiura + |
868 |
9 |
1,0 |
|
Uncinarias |
|||
|
P.f. + T. trichiura +Uncinarias |
868 |
3 |
0,3 |
|
Plasmodium vivax (P. v.) |
|
||
|
P. v. + A. lumbricoides |
1.983 |
58 |
2,9 |
|
P. v. + Uncinarias |
1.983 |
23 |
1,2 |
|
P. v. + T. trichiura |
1.983 |
21 |
1,1 |
|
P. v. + S. mansoni |
1.767 |
6 |
0,3 |
Table 3 Prevalence of coinfection in the general population
The prevalence between helminth and Plasmodium coinfection was 17.2%, with a higher occurrence of Plasmodium spp. and A. lumbricoides followed by Plasmodium spp. and T. trichiura. Specifically in P. falciparum the highest proportion of coinfection was registered with W. bancrofti, A. lumbricoides, S. haematobium and hookworms. In the case of P. vivax, it was with A. lumbricoides. Multiple infections were also found between helminths and P.falciparum, as is the case of 45 individuals with malaria plus infection with five different intestinal helminth species (Table 4). In the articles based on the population with malaria, a global prevalence of helminth of 52% was found, being higher those that occurred between Plamodium and A. lumbricoides, Uncinarias and T. Trichiura (Table 4).
|
Species |
Evaluated |
Positive |
Prevalence % |
|
Plasmodium spp. |
3.302 |
2.287 |
69,3 |
|
Plasmodium falciparum |
1.015 |
558 |
55,0 |
|
Plasmodium vivax |
552 |
435 |
78,8 |
|
P. falciparum + P. vivax |
552 |
22 |
4,0 |
|
Helmintos global |
3.302 |
1.718 |
52,0 |
|
Kato-Katz |
1.72 |
790 |
45,9 |
|
Sedimentation with formalin-ether |
1.291 |
755 |
58,5 |
|
PCR para Helmintos |
94 |
46 |
48,9 |
|
Plasmodium spp |
|||
|
P. spp. + A. lumbricoides |
2.77 |
1.284 |
46,4 |
|
P. spp. + Uncinarias |
2.052 |
1.004 |
48,9 |
|
P. spp. + T. Trichiura |
2.343 |
627 |
26,8 |
|
P. spp. + S. stercoralis |
844 |
229 |
27,1 |
|
P. spp. + S. Mansoni |
292 |
8 |
2,7 |
|
Plasmodium falciarum |
|||
|
P. f. + Uncinarias |
557 |
156 |
28,0 |
|
P. f. + A. lumbricoides |
754 |
136 |
18,0 |
|
P. f. + S. stercolaris |
848 |
89 |
10,5 |
|
P. f. + T. Trichiura |
754 |
169 |
22,4 |
|
Plasmodium vivax + |
|||
|
P. v. + S. stercoralis |
94 |
12 |
12,8 |
|
P. v. + Uncinarias |
94 |
11 |
11,7 |
|
P. v. + A. lumbricoides |
94 |
2 |
2,1 |
Table 4 Prevalences in the population with malaria
In seven studies that determined the prevalence of thick gout malaria in 4,995 pregnant women, a prevalence of Plasmodium-intestinal helminth coinfection of 15.4% (95% CI = 14.4-16.4) was found, with studies reporting co-infection in a range between 7.7% (95% CI = 4.9-10.5) and 18.7% (95% CI = 17.0-20.4) (Figure 3); being more relevant the coninfection of Plasmodium with A. lumbricoides with a prevalence of 8.0% (95% CI = 7.0-9.0), and unicinary with 12.4% (95% CI = 11.5-13, 4).
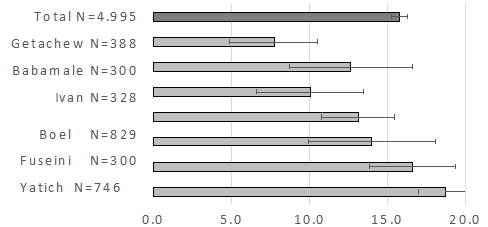
Figure 3 Meta-analysis of the prevalence of malaria (thick blood film) and intestinal helminths (Kato-Katz or Formol-ether) in pregnant women.
Similarly to what was found for pregnant women, in 25 studies that determined the prevalence of malaria with thick gout in 23,680 children, a prevalence of Plasmodium-intestinal helminth coinfection of 17.1% was found (95% CI = 16.6-17, 6) (Figure 4).
Malaria is a disease that can occur in association with different types of helminths, especially with soil-transmitted helminths or intestinal helminths (A. lumbricoides, T. trichiura and Uncinarias) which infect a third of the world population,5 or schistosomes such as S. haematobium and S. mansoni which are endemic helminths in tropical areas especially in Africa and East Asia and co-infections have been associated with P. falciparum.13
Coinfection between helminths and malaria occurs as a result of similar life cycles and environmental factors conducive to the transmission of both, such as the presence of malaria in the population near water sources where Anopheles spp. reproduces, which can be associated with the presence of helminth eggs and larvae in these sources, which many people can use for their consumption or domestic activities.12 To this must be added insufficient material living conditions, or the concentration of the population living in poverty in endemic areas for both types of infection, among other frequent situations in Africa, South America and East Asia.
Global prevalence of malaria
In this review, a malaria prevalence of 41% was found in the three indicated regions, this can be explained taking into account the world malaria report carried out by the WHO in 2017, which reported 216 million cases of malaria, in this report Africa reported 194 million cases, followed by East Asia with 14.6 million and South America with 875 thousand reported cases.14 The global distribution of malaria lists a prevalence in South America between 10 and 11%, in East Asia it is considered between 11 and 50%, while Africa has a prevalence greater than 50%15 corresponding to the continent with the highest number of reported cases.
It has been considered that P.falciparum and P.vivax are the Plasmodium species implicated in most cases of malaria,15 a situation that is confirmed by the prevalence of both species in this review, where 36.4% were found for P. falciparum and 11.6% for P.vivax. This result is also consistent with the number of malaria cases worldwide; For example, in Africa 99.7% of malaria cases were caused by P. falciparum, in East Asia P. falciparum corresponds to 66% of cases and P. vivax to 34%, while in the region of the In the Americas, the situation is the opposite since P. vivax corresponds to 64% of the cases.14
Global prevalence of helminths
In this review, the prevalence of helminths was 43.4% in which the majority of helminthiasis cases correspond to soil-transmitted helminths. Soil-transmitted helminths are considered to be responsible for the majority of helminthiasis cases in the world, only in Africa the prevalence of soil-transmitted helminths is 24.7%16 and in the world almost 24% of the population is infected by Ascaris lumbricoides; Trichuris trichiura and Hookworms (Necator americanus and Ancylostoma duodenale).5 It is estimated that A. lumbricoides is present in approximately 1,450 million people in the world, the Hookworms in 1,300 million and T. trichura in 1,050 million people.17 In this review, the distribution of A. lumbricoides is different from that reported worldwide since a prevalence of 10.7% was found, lower than the Uncinaries (15.5%), this difference can be explained because most of the population included in this review come from the African continent where these species present prevalences of 16.5% for Uncinarias, 6.6% for A. lumbricoides and 4.4% for T. trichiura.16
Schistosomiasis is an acute and chronic parasitic disease caused by trematodes of the genus Schistosoma and in this review it corresponds to the most prevalent helminth species after soil-transmitted helminths. In the world, schistosomiasis affects approximately 206.5 million people18 and the species of helminths are distributed throughout the tropical area, where Africa presents the majority of cases of infection and is the only continent that presents circulation of S. haematobium19 this would explain the fact that this species was the most prevalent in this review with 19.9%.
Prevalence of coinfection between helminths and malaria and its effects on human health
Helminths and Plasmodium spp. share the same geographic distribution and can affect the same population, cases of coinfection between malaria and helminths occur mainly in the tropics such as South America, Africa and East Asia,20 this explains why in this review it was found that the prevalence of relatively high coinfection with 17.2%, where the evaluated population come from these areas. In addition, the analysis by species showed that the highest prevalence of coinfection found in this review was between P. falciparum - A. lumbricoides and P. falciparum - S. haematobium, which could be attributed to the epidemiological nexus of these agents, which are distributed in a similar way in the same tropical territory.20 Regarding the impact of helminth-malaria co-infection on human health, there are several studies that have contradictory results. Malaria and soil-transmitted helminth infections contribute to the prevalence of anemia, being more severe in individuals who are simultaneously infected with malaria and helminths.21-23
In contrast, other studies have reported that coinfection between malaria and intestinal helminths does not affect hemoglobin values; however, hookworms are known to be associated with anemia due to their hematophagous character, as well as schistosomes due to their invasion into tissues and damage to multiple organs.24,25
Immunologically, it has been found that co-infection between helminths and malaria have a different effect on cytokine production and cellular response to single infections by both parasites. During co-infections with malaria, an immunomodulatory effect of helminths has been reported, a potential mechanism would be the production of IL-10 by helminth infection, which could protect against severe malaria.26 Bwanika et al. Found that IL-10 levels were higher in subjects coinfected by malaria and helminths compared to subjects with a single P. falciparum infection;27 Other studies indicate that the production of IL-10 favors the sequestration of parasitized red blood cells, which is why it can be protective against malaria, reducing the risk of complications.7
Coinfection between malaria and helminths can also cause a change in the immune response from Th1 to Th2, a polyclonal activation of B cells, and the production of cytokines such as IL-4 and IL-6, increasing IgE synthesis.28 Consequently, coinfection between malaria and helminths appears to increase serum IgE levels; this elevated IgE concentration was associated with a low P. falciparum parasite load in subjects with coinfection compared to subjects with malaria alone.29 During coinfection by helminths and malaria, helminths have been found to have a protective factor against malaria complicated by stimulating an inflammatory response.29
Populations most at risk of coinfection between helminths and malaria
Taking into account that the population included in the study comes from tropical countries, it can also be considered that people living in rural areas where the economy is based on agriculture have a greater risk of acquiring co-infection between helminths and malaria;30 due to a greater risk of contact with the vector and infective forms of helminths, since these areas are characterized by greater stagnation of water, dense bushes around houses, lower level of education, poverty and lack of preventive measures effective against malaria, or the type of housing that predominates in rural areas, being made of planks, cracks or other characteristics conducive to the vector.31
School-age children from tropical countries such as Africa and South America have a higher prevalence (36%) of coinfection between helminths and malaria, compared to the adult population (13.4%).32 This added to the fact of having greater risk behaviors such as contact with the ground, swimming or walking in fresh water such as rivers and lakes (risk behavior for Schistosoma spp), consumption of uncooked food, untreated water and greater risk for other conditions health problems such as malnutrition.6 For example, Righett et al found that early school-age children living in remote rural areas are at increased risk of coinfection between P. falciparum and Uncinarias due to poor hygiene, ecological conditions suitable for the life cycles of the parasites and difficult access to healthcare facilities for people living in this environment.32
Another population at risk of helminth infection and malaria are pregnant women due to the immunosuppressive nature of pregnancy and some frequent risk behaviors in women from poor areas, such as a diet low in iron and other nutrients.33 For this reason, pregnant women presented a high prevalence of helminth/malaria coinfection, which has been shown in analytical studies that have compared this group with women who are not pregnant,32 increasing the risks to the health of the mother and the mother. The newborn as an increased risk of anemia, premature pregnancies and low birth weight.34
A high prevalence of coinfection was found in a low number of studies, which shows that the study of interactions between Plasmodium and helminths constitutes an underdeveloped area in parasitology. Despite the high magnitude of malaria and helminths in America, studies of coinfection in the region are scarce, which constitutes an obstacle to avoid its clinical and epidemiological effects, while preventing the development of public policies for parasitological control in endemic areas. It is necessary to design health programs to prevent and control malaria and helminthiasis, and in the Latin American case, increase epidemiological investigations on the magnitude of coinfection, etiological studies to identify the factors and groups at higher risk, as well as the design and evaluation of interventions aimed at both health problems in endemic areas.
None.
None of the authors declare a conflict of interest for the publication of this manuscript.

©2021 Agudelo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.