Journal of
eISSN: 2373-437X


Review Article Volume 3 Issue 1
1Department of Veterinary Medicine, Ahmadu Bello University, Nigeria
2Department of Community Medicine, Bingham University, Nigeria
3Jigawa Research Institute, Nigeria
4Department of Veterinary PulicHealth and Preventive Medicine, Usmanu Danfofdio University, Nigeria
5Department of Medicine, Bayero University, Nigeria
Correspondence: Ibrahim S, Department of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
Received: November 26, 2015 | Published: January 18, 2016
Citation: Ibrahim S, Abubakar DSUB, Usman A, Muhammad FU, Musa GA (2016) Preliminary Study on the Prevalence of Bovine Tuberculosis and Risk Factors Among Pastoralists in Gombe State, North Eastern Nigeria. J Microbiol Exp 3(1): 00081. DOI: 10.15406/jmen.2016.03.00081
A cross-sectional study was conducted in GombeState (North- eastern, Nigeria) from March, 2011 to April 2013 to estimate the prevalence of bovine tuberculosis (BTB) based on single caudal intradermal tuberculin test (SCITT), questionnaire and an abattoirs survey. The study was conducted among 250 households (100 household with a history or confirmed case of pulmonary tuberculosis and 150 households who had no history or confirmed case of pulmonary Tb), who raised 217 herds comprising of 2,245 herds of cattle. Questionnaire administration and Bioline® analysis of sputa collected from all pastoralists who were suspected to have TB and were smear positive were also conducted. Herd prevalence of SCITT was 13.87% and was higher than in herds of the negative households. At least one human Tb case was observed among 25% of the households interviewed, whereas 48% had reactor cattle. However, there was no statistically significant association (P>0.05) between the human Tb cases observed and the presence of reactor cattle. Similarly, fourty (40) isolates of Mycobacteria spp were obtained from the cultured sputa collected from the pastoralists, and were subjected to Ziehl-Nelson (ZN) and Bioline® analysis, where sixteen (16) of the (40%) were found to be M. tuberculosis complex (MTC) and the remaining 24 (60%) were members of Non- tuberculous Mycobacteria (NTM). However, a significant difference (p>0.05) was observed among the cattle age group, cattle over five (5) years were seen to have higher odds for tuberculin reactivity compared to those below the age of five (5). Out of the total cattle population (57,943) subjected to post mortem examination at the abattoir, 205 were found to be positive for gross Tb lesions, where a large proportion (50%) of the lesions were recorded in the respiratory pathway, digestive system (21%) and prescapular lymph nodes (18.6%). In spite of all these findings, it was observed that 70% (175 out of 250) of the respondents had knowledge of the existence of the disease but did not know the zoonotic implication. Also, all the potential risk factors of the disease transmission between cattle and human (milk consumption, livestock husbandry and presence of Tb positive cattle) were not statistically significantly (P< 0.05). In conclusion, it was observed that cattle belonging to the pastoralists who were positive for Mycobacterium species were more affected than those who were negative and lack of zoonotic awareness of the households. Therefore, further study to differentiate the MTC (Mycobacterium tuberculosis complex) using molecular analysis, collaboration between physicians and veterinarians and creation of awareness about the zoonotic nature of the disease were recommended.
Keywords: Mycobacterium tuberculosis, bovine tuberculosis, pastoralists, Gombe state, north eastern Nigeria, veterinarians, Mycobacterium bovis
Tuberculosis (TB) is known to be one of the most important threats to both human and animals health causing mortality, morbidity and economic losses.1 In humans, tuberculosis caused mainly by M. tuberculosis, some mortalities and morbidities due tuberculosis are caused by bovine tuberculosis (BTb). BTb is an infection diseaseof cattlecaused by Mycobacterium bovis (M. bovis) that affects the productivity of livestock industry in the developing countriesjointly with other disease. It is a zoonotic disease transmitted tohuman by aerogenous route and/or through consumption of contaminated milk and other cattle products.2
In Nigeria, the human population is growing causing increaseddemands on animal productssuch as milk and meat. This in turn caused and is still causing intensification of livestock keeping integrated with genetic improvement.3 BTb is a disease of intensification and cross breeding between exotic and local cattle breeds to increases high potential milk yield, could probably put the people especially those that drink raw milk to be potentially under the risk of beingof being infected with M. bovis.
The globalprevalence of human TB due to M. bovis has been estimated at 3.1% of all human TB cases, accounting for respectively 2.1% and 9.4% of pulmonary and extra pulmonary TB cases.4 In countries where BTb in cattle isstill highly prevalent, pasteurization of milk is not widely practiced and/or milk hygiene is insufficient, usually an estimated 10% to 15% proportion of human tuberculosis is considered to be caused by BTb.5
The economic loss caused by this disease is enormous and great in animal production. Infected animals losses 10-15% of their productive efficiency. Direct losses dueto the infection become evident by decrease in 10 - 18% milk and 15% reductionin meat production6 apart from effectson animals production, it has also significant public health importance.7 Presently, the disease in humans is becoming increasingly important especiallyin developing countries, as human and animal are sharing the same micro-environment and dwelling premisesespecially in ruralareas, and susceptibility of AIDS patients to tuberculosis.8
In developing countries such as Nigeria, the socio - economic situation and low standard ofliving, makes both animals and humans to live in the same area, thereby contributing in Tb transmission between human to human and human to cattle or vice versa. Increased numbers of infected human individual willalso leads topossibility oftransmission tocattle toincrease. Studies previously done in Nigeria reported isolating M. bovis frommilk samples and tuberculous lesions collected from tuberculin positive cattle and sputum sampled from persons working in tuberculin positive herds. However the baseline information on the true prevalence ofBTb in both cattleand humans is lacking in the study area. Therefore, the objective of this study was to undertake preliminary investigation of theprevalence of BTb and the risk factors associated with thedisease amongthe pastoralist and their cattle in Gombe State
The study was conducted from March 2010 to April 2013 in the three senatorial districts of Gombe State. Gombe state is located between latitudes 9° 30̍’and 12° 30’ North and longitudes 8° 45’ East. It lies in the centre of the North-East geopolitical zone of Nigeria. It shares common boundary with all the other states in the zone, namely Adamawa, Bauchi, Borno, Taraba and Yobe. The state occupies a total land area of about 20,265 sq.km. The vegetation of the state is generally guinea savannah grassland with concentration of woodland in the southeast and southwest. There are two distinct seasons the dry season (November to March), and wet season (April to October). Average rainfall is 850mm.9 The state was created on the 1st October, 1996 with a total of eleven Local Government areas. It has a population of 2.03 million people according to 2006 National Census.
The Local Government areas are; Dukku, Gombe, Kwami, Nafada and Funakaye (Gombe North), Balanga, Billiri, Kaltungo, Shongom (Gombe South) and Akko and YamaltuDeba (Gombe Central). Estimated cattle population in Gombe State is 800,000 heads of cattle according to the directorate of veterinary services of the ministry of agriculture Gombe State.
The hospitals located in each of the selected area were used, also the areas were selected due, to the fact that people are living in close association with their cattle in a homogenous condition, and thus the dynamics of Mycobacterial disease transmission between cattle and the people living with them can be clearly investigated.
Study design
A cross -sectional study was conducted on selected 250 households, consisting of 100 Tb positive patientsand 150 Tb negative individuals. The study herd size was 20 heads of cattle. The total herds comprised of 2,245 heads of cattle.
Study subjects
The study was conducted among the pastoralists using cross sectional study to determine the prevalence of BT bin the study area. Patients having cattle were identified at their respective clinicsand traced back to their hamlets being guided by health workers. A human Tb case was defined as a Tb positive patient diagnosed and confirmed at respective hospital, negative lists were purposely selected from villages where these Tb patients live, within 5km radius, on the basis of absence of any member of the family that has shown any clinical sign suggestiveor diagnosed as Tb positivefor the last five years. All cattle above 1 year old in herds’ owned by both Tb positive and negative pastoralists were tested using caudal intradermal tuberculin test. Both groups of pastoralists were interviewed to assess their awareness about zoonotic importance of bovine Tb.
All the cattle included in this study were local zebu breeds kept under extensive pastoralist management system, information related to each tested cattle (such as sex age, body condition score (BCS) were collected and recorded at the time of the tests. Ages of the cattlewere determined according to DeLahunta and Habel (1986). The body condition of each of the study cattle was scored usingguidelines established by Nicholson and Butter worth (1986). Based on the observation of anatomical parts such as vertebral column, ribs and spines, the cattle were classified as lean (score 1- 2), medium (score 3) or fat (score 4 -5).
Single caudal fold
The test was conducted on 2,245 heads of cattle. 0.1ml of bovine PPD obtained from Netherlands was injected intradermally in the caudal fold at the base of the tail; approximately 72hrs after initial injection, the injection site was visualized and palpated for any swelling or sign of inflammation of the area. The cattle were positive if any sign of inflammation was detected.
Interview of cattle owners
A total of 250 household members (100 were confirmedcase of TB and 150 were negative). These individuals were interviewed by their local language. The interview consisted of closed and open ended questions which address their knowledge, attitude and practices for the pastoralist in relation to Tb in human and cattle.
Culturing of sputum
A total of 250 sputa samples were collected in sterile universal tubes from smear positive pulmonary Tb patients using leak proof disposable plastic materials. Two sputum samples, one on the spot and one overnight and were later pooled together. Thesputum sample were processed (decontaminated and neutralized) for Mycobacterium culturing according to the standard operating procedure described by WHO (2008). Thereafter, 100µl neutralized sample was inoculated on to two slants of Lowenstein Jensen (LJ) media, onesupplemented with pyruvate and the other with glycerol, and incubated at 37 °C for up to 5-8 weeks. Growth of Mycobacterium was monitored every week for up to 8week. Samples negative for AFB after 8weeks of growth were considered negative.
Culturing of tissues from cattle
200 tissues samples were also obtained from the abattoir after detailed postmortem examination. All tissues suspected to have lesions compatible with Tb were collected and cultred on solid media (Lowenstein-Jense) media enriched with pyruvate and glycerol and incubated at 37°C for 8weeks and growth was monitored for AFB. Samples negative for AFB after 8 weeks were considered negative.
SD bioline TB AgMPT64 analysis
A total of 40 isolates were obtained from the cultured sputa samples. The isolates were subjected to SD Bioline® TB AgMPT64 analysis, according to the manufacturer’s instructions. This test consisted of test cassette which consists of a sample pad, a gold conjugate, a nitrocellulose membrane, and an absorbent pad. Mouse monoclonal anti-MPT64 was immobilized on the nitro cellular membrane as the capture material (test line). Another antibodies, which recognized another epitope of MPT64, conjugated with colloidal gold particles were used for antigen capture and detection in a sandwich type assay. The cassette has a letter T and C as test line and control line on the surface of the case. Positive results produced red to purple band. In the absence of MPT64, there is no line in the test region. Briefly, 5-7 colonies were emulsified in about 200µml sterile buffered saline, then, 100µml of the suspension added into the sample well and allowed to stay for 15minutes before being red positive result is indicated by the presence of 2 color bands (one control band and one test band).The presence of only one control band within the result window indicated a negative result. Faint color band was recorded as a weak positive and the sample was retested.
Molecular identification: All cultures obtained from samples (necropsy and human sputum) were collected and then subjected to molecular analysis using PCR-based molecular technique; known as Hain assay Test (Genotype MBTC kit). The following procedure was based on the manufacturer’s instructions.
Ethical consideration
The purpose of this study was explained to the cattle owners as well as all the human patients’ whosesputa were collected and informed consent was obtained.
Data analysis
The collected data was entered into Microsoft excel spread sheet. Statistical analyses were performed using statistical package for social science (SSPSS) version 16 (2007) packages. A percentage was used to calculate the prevalence of TB in both groupsatherd and individual cattle level. The presence of statisticalprevalence of bovine Tb was analyzed using chi- square test. .Information generated through questionnaire was analyzed using percentages. In all cases 95% confidence internal (CT) and P<0.0.5 was considered statistically significant.
Herd prevalence
From a total of 217 herds tested, 311(13.87%) of them contained at least one reactor cattle for SCITT test. The result on the effect of herd size andmanagement on the herd prevalence of BTb are presented in Table 1. Animal prevalence was significantly higher in cattle owned by pastoralists with Tb than cattle owned by Tb free individuals (Table 1).
|
Variables |
Tb positive (%) |
Tb negative (%) |
Total |
|
Herds |
120 (55.29) |
97 (44.70) |
217 |
|
Individual animal |
311(13.85) |
1934 (86.14) |
2245 |
Table 1 BTb prevalence in herds and cattle owned by pastoralists with and without Tb
C.I: 95%, P- Value<0.05.
Individual animal prevalence
Disease prevalence atanimal level by SCITT was 18.5%. The prevalence varied significantly among groups (p< 0.05) Table 1.
Postmortem examination results
Prevalence of 14% of bovine Tb was observed on the basis of detailed post mortem examination. This was indicated in Table 2, the highest proportions of 50% of the gross lesions were observed in the thoracic cavity. The results of the infection rate of tuberculous lesions among slaughter cattle with different factors were presented in Table 3 &4.
|
Hospital |
Total number of sputum |
Culture positive |
Bioline positive |
|
Gombe |
30 |
7 |
3 |
|
Kaltungo |
20 |
3 |
1 |
|
Bajoga |
20 |
10 |
2 |
|
Zambuk |
50 |
20 |
10 |
|
Total |
120 |
40 |
16 |
Table 2 Human sputa cultured and anaylysed by bioline
|
Variables |
Number tested |
Number tested |
|
Herd size |
||
|
< 5 |
1228 |
215 (17.51) |
|
10-Jun |
757 |
85 (11.23) |
|
> 10 |
260 |
11 (4.23) |
|
Management |
||
|
Poor |
1455 |
209 (14.36) |
|
Good |
790 |
102 (12.91) |
|
Age |
||
|
< 2 |
57 |
4 (7.01) |
|
4-Feb |
509 |
107 (21.02) |
|
7-Apr |
1135 |
146 (12.86) |
|
>7 |
294 |
44 (14.97) |
|
Sex |
||
|
Male |
611 |
55 (9.00) |
|
Female |
1634 |
256 (15.67) |
|
Body condition score |
||
|
Fat |
774 |
40 (12.86) |
|
Medium |
737 |
55 (17.68) |
|
Emaciated |
734 |
216 (69.46) |
Table 3 Effect of different risk factors on herd prevalence of BTb
|
Organ |
Number of Tb lesion |
Percentage |
|
Mediastinal lymph node |
1015 |
12.51 |
|
Tracheobronchial lymph node |
856 |
10.55 |
|
Mesenteric lymph node |
1704 |
21.01 |
|
Medial retropharyngeal lymph node |
533 |
6.57 |
|
Lung |
2355 |
29.3 |
|
Submandibular lymph node |
1649 |
20.33 |
|
Total |
8112 |
100 |
Table 4 organ distribution of Tb lesions of cattle slaughtered at the abattoir
Culture and isolation of mycobacteria from bovine tissues from the abattoirs
The 200 bovine tissue samples collected were cultured on solid media (Loweinstein - Jensen media) enriched with pyruvate or glycerol. Of the 200 samples collected and cultured, 138 (67%) were negative or had no growth on them after 8 weeks of incubation, 62 (32.5%) were positive based on cultural characteristics and Zeihl-Neelsen Stain technique. Approximately, 25 (12.5%) of the samples were found to be contaminated (Table 5). The positive acid fast bacilli (62/200) were then subjected to Bioline® (SD TB AgMPT 64 rapid) analysis, this classified them into either Non tuberculous Mycobacteria (NTM) or Mycobacterium tuberculosis complex (MTBC). This analysis yielded 18 as MTBC, while the remaining seven were characterized as NTM (Table 5).10
|
Type of specimen |
No. of samples collected |
AFB Positive (%) |
Culture Positive (%) |
Bioline Positive (%) |
|
Sputum |
250 |
105(42) |
74(29.6) |
40(16) |
|
Tissue |
200 |
62(32.5) |
25(12.5) |
18(9.0) |
Table 5 Specimen reactivity to various tests (Smear, Culture and Bioline ®) Positivity
Culture and isolation of mycobacteria from human sputum
Two hundred and twenty five (250) smear positives sputum were collected from Gombe State. All the sputum samples collected were then cultured on solid media (Lowein-Stein-Jensen) media supplemented with glycerol and pyruvate. Of the 250 sputum samples, 74 (29.6%) were positive based cultural characteristics andZ-N staining technique, 98 (39.2%) were either contaminated or the culture had dried-up, while 78 (31.2%) were negative or had no growth on them after 8 weeks of incubation (Table 5). The 74 cultured and smear positive samples were then subjected to Bioline® (SD TB AgMPT 64 rapid) analysis and 40 (16.0%) were classified as MTBC, and the remaining 34 (13.6%) were NTM (Plate 1 & 2).
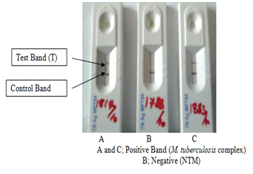
Plate 1 Differentiation of M. tuberculosis complex and non-tuberculous Mycobacteria by BIO-LINE SD Ag MPT64 tuberculosis test.
Questionnaire
The result of the interview conducted revealed lack of awareness of the pastoralists about the zoonotic nature of the disease, but showed that 70% of them were aware of the BTb (Table 6).
|
Variable |
Yes (%) |
No (%) |
|
Heard about BTb before |
175 (70%) |
75 (30%) |
|
Know transmission of BTb from cattle to human |
35 (14%) |
215(86%) |
|
Consumption of raw milk |
25 (10%) |
225 (90% |
|
Confirmed human Tb in households |
100 (40%) |
50 (60%) |
Table 6 Public health awareness on zoonotic importance of bTb
Both Tb positive and negative pastoralists had less awareness of zoonotic importance of the disease, while the respondents were aware of the transmission of the disease from human to cattle and vice-versa respectively. It was observed that 90% (225 out of 250) of the respondents consume raw orunboiled soured milk. 70% of the pastoralists that had patients in their family owned reactor cattle in their herd. Significant association was obtained between the pastoralists with Tb patient and reactor cattle in their herd.
In this study, we evaluated the presence of BTb and its risk factors among the pastoralists in Gombe state, Nigeria. This was achieved by including TB positive and TB negative pastoralists and the cattle owned by bothgroup were investigated. The individualanimal prevalence recorded by this study washigher than the report by Cadmus et al.,11 in one cattleherd in Ibadan Nigeria. The increment might be attributed to the fact that the prevalence’s of BTb is influenced by breed of cattle also the number of animal screened were far greater thanthe one herd screened by Cadmus et al.,11 Estimates of the sensitivity of tuberculin tests ranged from 68% to 95%12 and it has long been known that the sensitivity of tuberculin test affected by the potency and dose of tuberculin administered, the post infection interval, desensitization, postpartum immune suppression and observer variation6 to this effect doubtful reactors were added to thepositive reactorsin calculating the overall prevalence due to the fact that sensitivity of tuberculin test is low which may lead to miss infected animals under the actual prevalence of BTb. Studies by Ameni et al.,13 indicated that infections of cattle withgastrointestinal parasites such as Fasciola and Strongyle, compromised the immune response to tuberculin test by shifting the immunity from Th1 to Th2 response there by promoting the emergence of false positives.
In Nigeria, studies by Danbirin et al.,14 indicated prevalence of 11.8% in cattle owned Tb positive herdsman after tracing back. Thetrend of high prevalence of TB among human patients in Nigeria is similar to the trend observed among cattle populations; thus indicating a relationship between the disease in human and infection in cattle.15 The presence of higher TB reactor cattle in cattle owned by Tb positive pastoralists than Tb negative pastoralists suggest that either of them could be a source of infection for the other as the disease may be cyclical (cow- to man and man- to cow).4
Furthermore, old cattle above 7 years commonly show lower reaction to tuberculin test16 and in this study, the area hadfewer cattle that were above 7yrsthanyoungcattle, this might be additional factors that contribute to explain the apparently low prevalence of bovine tuberculosis.
According to the result of this study, it was observed that the infection is more prevalent in female cattle than male cattle and cattle having medium BCS. Young adult cattle (>2<5yrs) were found more susceptible followed by adult cattle (>5<9 yrs). Tuberculin reactivity was significantly affected by the body condition of the cattle. This could be because the tuberculin reaction is dependent on immune competence, which in turn may be associated with the physical condition of the animals such that animals with better physical condition are immune, competent and therefore, give a better reaction to tuberculin. But animals with poor body condition could be immune compromised and hence may not react to tuberculin although they might have been infected by Mycobacterium .10 This is in line of agreement with the work of Lackech et al.,17 in which the disease is found to be more prevalent in young adult and medium body conditioned cattle, but this finding was contrary to the findings of Shehu18 in Nigeria who reported that male cattle had higher chance of being positive because of its occupation as male cattle in the herd. According to Nega et al. analysis for the effect of risk factors revealed that the animal levelof prevalence of BTb increased with age up to the age of 7 years and was then observed to decease slightly. This findingis consistent with other reports.7,19,20 Further, Tizard21 stated that lowered response tointradermal tuberculin test in older animalsis due to theimmune depression resulting from old age.
Although the number of M. bovis positive samples was low, the habit of polling milk still pose a public health danger to the consumers of unboiled milk as one cow with tuberculous mastitis can excrete enough viable tuberculosis bacilli to contaminate milk and milk products.22 According to the results of the current study, 90% of the interviewed households in the study area used raw or soured milk. Similarly, high proportion of cattle raising families were reported to have the habit of drinking raw milk by studies conducted in Nigeria.13,23,24 Consumption of unpasteurized fresh and soured milk potentially infected with M. bovis was found to cause milk borne infection with Tb. Such risk was reported to be the main cause of non-pulmonary Tb and is high in areas where BTb is common and uncontrolled.25
The association of human tuberculosis in the households and presence of a reactor cattle observed in this study was found to be significant. Similarly, different studies conducted on the assessment of public health implication of BTb in different parts of Nigeria revealed statistically significant association between reactor cattle and confirmable Tb patients among family members of cattle owners.14,15
The poor understanding of the cattle owners on BTb observed in this a study could also signify the public health implication of BTb. Only 14% of cattle owners interviewed understood that BTb is zoonotic, while 70% of the respondent did not know that cattle could have Tb. This agrees with report from Cameroon, which indicated 81.9% of cattle handlers know BTb; however, with 67.9% of them know BTb is zoonotic.26
Largest proportion (90.56%) of gross lesion was detected in thoracic cavity followed by head region 5.85% and abdominal cavity (3.56%). This was in agreement with the findings of Ameni & Wudie27 who found 72% of the gross lesions in thoracic cavity. Regassa et al.,28 and Tigre et al. also detected 50% and 48.4% of Tb lesion, respectively in respiratory pathway (lung and associated lymph nodes). These indicate that there is need for special attention on the lungs and associated lymph nodes during post mortem examinations for Tb lesions.
The study has shown a high prevalence of Non tuberculous Mycobacteria (NTM) otherwise known as environmental Mycobacteria in cattle studied to be (7.5%) and 13.6% in humans, which signifies environmental contamination. Cattle could have acquired the infection through contaminated environment during grazing or at water sources. The high prevalence of NTM seen in this study in both cattle and human is even lower than the previous reports in Jos, Nigeria by Mwak et al.,29 who reported a prevalence of 23.08% in humans, and also in Tanzania by Shirima et al.,30 and Durnez et al.,31 reported prevalence of 6% and 10% respectively in cattle. Similarly, in their study Mdegella et al.,32 and Durnez et al.,31 found also high prevalence of 14% and 19% of NTM in milk samples. Although this study did not investigate milk and its products, it is a known practice in the study area that consumption of unpasteurized milk is a very common practice; these might expose milk consumers in the study area to a great risk of infection. The result of this study indicated that, M. bovis (4%) and M. tuberculosis (2%) were identified in slaughter cattle in the study area, indicating an important finding with economic and public health consequences. A study by Byrugaba et al.,33 reported that M. bovis was responsible for 3.1% of all forms of human TB worldwide.
In this study, various Mycobacterial species were isolated from lesions compatible with bovine tuberculosis in slaughtered cattle in the study areas. Mycobacterium bovis and Mycobacterium tuberculosis, isolated from samples collected and cultured from slaughtered cattle.. It is believed strongly that the main source of M. tuberculosis in animals (anthropozoonotic transmission), including cattle is from human suffering from active TB. Although M. tuberculosis is considered primarily a human pathogen, it has also been reported to be present in domestic and wild animals, most frequently living close contact with humans.34 Available literature suggests that humans suffering from active TB are the most probable source of M. tuberculosis in cattle have been described in the past but the firs unequivocal evidence of human - to - cattle transmission of M. tuberculosis confirmed by IS6110 restriction fragment length polymorphism (RFLP) analysis was not reported until 2005.35 In other studies M. tuberculosis has been isolated in cattle especially those in close contact with human who has or have active tuberculosis due to M. tuberculosis35,36 and are believed to be the source of M. tuberculosis in cattle. Other studies previously have claimed the prevalence of M. tuberculosis in cattle to have always not exceeded 1%35 but findings from this study, found the prevalence to be 2%. Recent studies have however revealed that the prevalence of M. tuberculosis in African and Asian cattle ranges between 4.7% - 30.8% in countries with high human TB incidences.37 The isolation of M. bovis and M. tuberculosis in slaughtered cattle confirms that there is zoonotic tuberculosis in the studied areas, which should be considered a public health concern especially among the pastoral communities that have strong attachments to their cattle for cultural, social and economic welfare.38
The majority of MTBC cases in our findings were caused by M. tuberculosis (12%), 2.8% as M. bovis and 1.2% were M. africanum in the human sputum samples analyzed. The proportion of M. africanum is low compared to previous reported studies done in some parts of Nigeria; Cadmus et al.,11 reported 13% in Southern Nigeria, while Waziri et al.,38 reported 10.8% in pulmonary patients in Zaria, Kaduna State. The reasons for the low prevalence in our study has not been deduced, but finding M. africanum which has been isolated in milk of pastoral cattle in Nigeria may suggests possible zoonotic transmission of this organism which was previously thought to be limited to humans.
In conclusion, this study indicated higher prevalence of BTb in cattle owned by tuberculosis positive pastoralists than tuberculosis negative pastoralistsand a lack of zoonotic awareness of the households. Although this study could not established the source of the infection whether it was from human to cattle or vice- versa, further study and establishment of collaborationbetween physicians and Veterinary Medical personals to trace back positive patients to get profile of their cattle and creation of awareness about zoonotic importance of the disease in the study area were recommended.
This work was supported by Agric Research Council and Federal Livestock Department, Abuja. The materials and financial support given by Zanklin Medical Centre Management, Abuja is highly acknowledged, and all the staff of Zanklin TB Research laboratory and the Department of Veterinary Services Gombe State, Nigeria.
Authors declare that there is no conflict of interest.

©2016 Ibrahim, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.