Journal of
eISSN: 2373-437X


Research Article Volume 10 Issue 4
1Unidad de Innovación en Diagnóstico Celular y Molecular. Avenida Sierra Leona, México
2Centro de Investigación Aplicada en Ambiente y Salud (CIAAS), Avenida Sierra Leona, México
3Departamento de Farmacia, Divisón de Ciencias de la salud, Universidad de Quintana Roo, México
Correspondence: Luz Eugenia Alcántara-Quintana, CIACYT, Avenida Sierra Leona Mexico, Tel +52 553 290 19 15
Received: September 01, 2022 | Published: September 15, 2022
Citation: Lopez-Mendoza CM, Leon-MartínezDL, Rodríguez AM, et al. Ozone as a disinfectant in laboratory surfaces against the SARS-cov-2 coronavirus. J Microbiol Exp. 2022;10(4):136-140. DOI: 10.15406/jmen.2022.10.00364
Introduction: The treatment of surfaces with ozone has become important due to the ease of the SARS-CoV-2 virus to reach places where it is not normally disinfected with chemical treatments. Ozone can be supplied from two sources: ozone generators and electrostatic air purifiers, both of which leave no residues that damage the environment. Ozone is highly effective against bacteria, fungi, mold, and virus inactivation. The objective of this work was to investigate the disinfection of surfaces naturally contaminated with SARS-CoV-2 and bacteria by using ozone plasma. Material and methods: We examined the disinfection capacity of ozone plasma against the SARS-CoV-2 and bacteria, through a study of natural contamination in situ. Amplification of specific genes by real-time reverse transcription-polymerase chain reaction of SARS-CoV-2 and microbiological culture of bacteria was performed before and after the disinfection process.
Results: SARS-CoV-2 was not detected in all assays; bacteria were not cultivable after disinfection with ozone plasma.
Conclusion: Disinfection with ozone plasma technology can be an alternative for their use in a shortage situation of others disinfects. Implications for the use of disinfection technologies of surfaces lab’s and the safety of laboratory personnel are discussed.
Keywords: ozone, disinfectant, SARS-CoV-2 coronaviruses
What all started as pneumonia cases in Wuhan, China, in December 2019, gave rise to a new pandemic announced in March 2020.1 The virus, SARS-CoV-2, causing COVID-19 disease is part of the Coronaviridae family, has a single-stranded positive-sense RNA (+ ssRNA) genome. This genetic material is contained within a nucleocapsid packed by an envelope associated with structural proteins such as membrane, spike, and envelope proteins.2 Transmission is by respiratory droplets, aerosols, direct contact with contaminated surfaces, and orofecal transmission3 (Figure 1). Symptoms may appear from 2 to 14 days after exposure to the virus and include fever; cough; loss of smell and/or taste, sore throat, diarrhea, etc.4
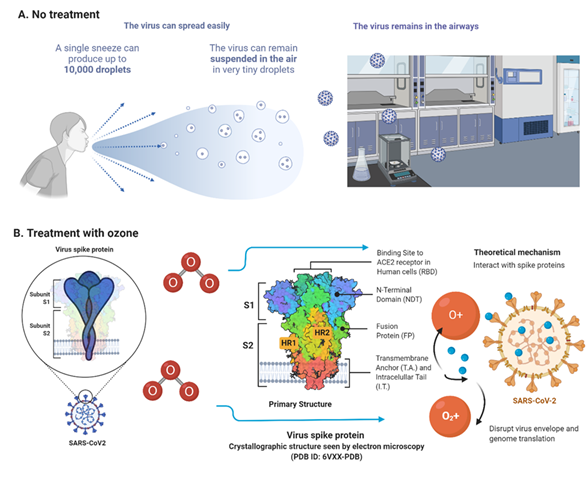
Figure 1 Physical laboratory spaces treated before and after with ozone plasma. A. Viruses remain in the air as droplets before treatment. B. After treatment with ozone, it interacts with the spike protein of the Sars-CoV-2 virus. Disrupt the virus envelope and preventing genomic translation. Created in biorender.com.
In Mexico, COVID-19 has a great impact, leaving so far 1.77 million positive cases with 150 thousand deaths. Specifically, in San Luis Potosi, the data are not at all encouraging, being in the first positions of cases nationwide with 28,400 cases and 1994 deaths, with more positive cases reported daily.5
It has been demonstrated that this virus can survive in the air and the environment for up to 9 days at a temperature of 22°C; additionally, its survival has been observed on material surfaces such as metal, wood, cardboard, and plastic.6,7,8
Ozone (O3) is a colorless gas, formed by three oxygen atoms that, in normal conditions in the environment, is decompounded into molecular oxygen and a reactive singlet of oxygen. Its abundance in nature is about 0.04 parts per million, most of it located in the stratosphere (90%). In water, the solubility of this gas is ten times greater than the molecules of molecular oxygen (49.0mL/100mL at 0°C). It's regular to find higher concentrations of this gas near the ground environments due to the density higher than air (2.14 kg/m3). Ozone with its oxidant power reacts with organic molecules in the parts of microorganisms' anatomy, which leads to the fungicidal, bactericidal and virucidal effect. Hence, ozone has wide applications in day-to-day life, like in water treatment, air purification, and medical therapies. On the other, ozone lacks the approbation of the Center for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) to be implemented in disinfection or sanitization in personal protection equipment and surfaces due to the high concentrations that are required that may lead in some secondary effects.
The treatment of surfaces with ozone has gained importance due to the ease with which it can reach places where it is not usually disinfected with chemical treatments. Ozone can be supplied from two sources: ozone generators and electrostatic air purifiers, both of which do not leave residues that damage the environment (Figure 2).
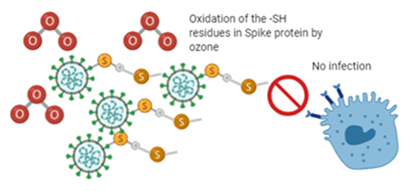
Figure 2 Proposal of the interaction of ozone with spike protein of the virus SARS-CoV- 2. Oxidation of -SH- residues of spike protein by ozone. Created in biorender.com.
Ozone has been highly effective against bacteria, fungi, mold, and virus inactivation, as long as it is not used on porous surfaces. Previously it has been observed that there are conditions that play a fundamental role in its effectiveness such as concentration, temperature, humidity, and exposure time with high variability among bibliographies, unfortunately, there is not so much information about this treatment on surfaces to eliminate SARS-CoV-2. 9-12
The ozone has fateful on naked and enveloped virus. Ozone on the enveloped viruses, disrupts the viral envelopes due to the phospholipid and lipoprotein peroxidation, leading to the contact of ozone also with the genetic material, causing its degradation. In the case of naked viruses, the nucleocapsid (that consists of proteins) is also affected by ozone and subsequent degradation of nucleic acids. That’s why ozone should be considered for surfaces and air sanitization against SARS-CoV- 2.
Considering the above and reports of SARS-CoV-2 survival on surfaces, the main objective of the present study was to i) detect SARS-CoV-2 RNA on high and low contact surfaces and ii) test the effectiveness of an ozone generator for use on inanimate surfaces for disinfection.
Sampling location: Sample collection was carried out in a laboratory of a research group, in which there is a flow of at least 20 people per day. Three different spaces were evaluated in this laboratory, which is shown in Table 1.
Sampling conditions |
Temperature (°C) |
Relative Humidity (%) |
General admission |
18 |
36 |
General laboratory |
19 |
39 |
Cell culture laboratory |
21 |
37 |
Table 1 Laboratories sampling locations
Sample collection: A total of 12 samples were collected from different sources of common use, before and after treatment, including i) general entrance door handle (metal); ii) disinfection table (wood); iii) work table cover (plastic) and iv) the surface of a biosafety hood (metal). Sampling was performed using sterile swabs with sterile PBS (Phosphate Buffered Saline), 1 mL in Eppendorf tube, vigorously swabbing the surfaces in an area of 10 cm2 on the previously mentioned objects. Subsequently, the volume of the Eppendorf tube with the sample was transferred to a 15 mL falcon tube containing PBS and taken directly to the laboratory for analysis.
RT-qPCR: The RT-qPCR technique (GeneFinder COVID-19 Plus kit) was used in a final volume of 20 mL per reaction, using primers and probes specific for the RdRp, E, and N regions of the SARS-CoV-2 genome. Samples were considered positive when they presented amplification for the three target regions, considering the cycle threshold (Ct) value to be less than 40. The QuanStudio 1 kit (Thermo Fisher) was used with the supplier's conditions.
Evaluation of environmental microorganisms: For this test, sampling was performed in different key points of the laboratory: crowded (entrance and general laboratory) and uncrowded (cell culture laboratory). Sterile Petri dishes with Blood Agar (AS) and Papa Dextrose (APD) were used to recover bacteria and fungi. For this purpose, the plates were left open, in duplicate, for an exposure time of 30 min. After this time, they were closed and incubated. The boxes with AS were incubated at a temperature of 37°C ± 1 °C, and the development was observed at 1 day, 4 days, and 7 days of incubation. For the boxes with APD, they were kept at room temperature (25 °C ± 1 °C) in a dark chamber and the development of microorganisms was observed for the same times as those with AS.
Ozone treatment. An ozone generator was used in the sampling areas for 10 to 15 min at 0.007 ppm ozone concentration measured with an environmental ozone detection equipment (Genetic®) detecting 1 mm particles.
Of the samples tested, 4 samples (33.33 %) (Figure 1) were positive for the presence of SARS-CoV-2 RNA. SARS-CoV-2 RNA was detected in samples collected from the laboratory corridor, the entrance handle, the disinfection table, and the plastic covering the work table in the general laboratory, with Ct values in the range of 6 to 26 for gene N. The controls used corroborated our procedure, being appropriate for their purpose. On the other hand, the samples taken from the biosafety hood were negative, due to the use it has to be constantly disinfected with 70% ethanol with 5 minutes of exposure.
The samples were collected from different materials, 4 from plastic (33.33 %), 6 from metal (50 %), 2 from wood (16.67 %). Of the 4 positive samples (Figure 3), they were before applying the ozone treatment, including surfaces: 2 (50 %) of plastic; 1 (25 %) of metal, and 1 (25 %) of wood. On the other hand, when these surfaces were exposed to ozone, no traces of SARS-CoV-2 RNA were found in our analysis, reducing the positive samples after treatment.
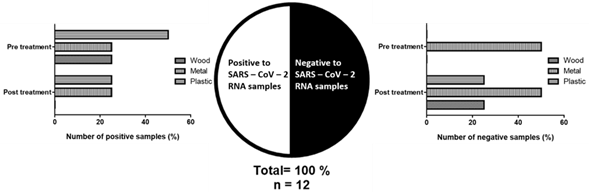
Figure 3 Samples collected positive for SARS CoV 2 RNA materials before ozone treatment. And positive samples for SARS-CoV-2 RNA in different materials before and after ozone treatment.
Regarding the sampling of other microorganisms, bacteria, and fungi, no presence or growth was found in the exposed boxes with different agars; the results are presented in Table 2, Figure 4.
Location |
Position 1 (CORRIDOR) |
Position2 (TABLE) |
Position 3 (Laminar flow hood II-B) |
|||||||||||||||
Agar |
Potato Dextrose |
Blood |
Potato Dextrose |
Blood |
Potato Dextrose |
Blood |
||||||||||||
Readings |
1D |
4D |
7D |
1D |
4D |
7D |
1D |
4D |
7D |
1D |
4D |
7D |
1D |
4D |
7D |
1D |
4D |
7D |
Number of colonies before treatment |
ND |
ND |
ND |
ND |
2 |
2 |
ND |
ND |
ND |
ND |
1 |
1 |
ND |
ND |
ND |
ND |
2 |
2 |
Number of colonies after treatment |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
Table 2 Microbiological analysis before and after treatment with ozone plasma
The results of the present study demonstrate the presence of SARS-CoV-2 RNA in different locations in a research laboratory, on high-contact and low-contact surfaces, although it is not yet proven that the presence of SARS-CoV-2 on surfaces is capable of triggering an infection process in the organism, the importance of maintaining contamination-free environments is considered of great relevance to eliminate the possible risk of contagion and spread of the virus. The particulate air during sampling had a size ≤ 1 mm, and in agreement with what has been reported in previous studies, particles in the air can be carried to other places and store the virus in particles of ≤ 5 mm6, and therefore increase the risk of transmission and permanence of the virus in the environment. It is important to note that if the virus was found on these surfaces, it is likely that the ambient air has a role in this result, highlighting its importance in terms of the risk of contracting the infection from aerosols in the absence of adequate personal protective equipment.13
Previous studies have reported the isolation of SARS-CoV-2 from indoor places such as health care facilities, bus terminals, and laboratories, as well as from open spaces such as parks, bus stops, or pedestrian walkways.14-17 In addition, detection of SARS-CoV-2 has been reported on high-touch surfaces, especially inside hospitals, suggesting that environmental contamination by the virus is possible due to people who have been symptomatic for less than 1 week, either in hospital beds or on nurse call buttons, cell phones, keyboards, monkeys, etc.18,19
There are experimental studies that corroborate the efficacy of the use of ozone in SARS CoV-2 disinfection and inactivation under different conditions34 made a model with pseudoviruses for the evaluation of ozone as a disinfectant for SARS–CoV–2. They found that ozone at a concentration on air of 1 000 ppmv with 30 min of exposure can decrease 99 % of the virus infectivity in surfaces.
Temperature and humidity represent two conditions necessary for virus stability on inanimate surfaces and the environment. Positive samples were collected under conditions of temperature in the range 18 - 21 °C and relative humidity of 36 - 39 %. Given the low sampling numbers, the data collected ignore the temperature-humidity relationship and virus permanence on surfaces, although other studies have reviewed these parameters in more detail. It is known that at relative humidities ranging from 17 to 80 % the virus can persist for days,20,21 added to this, it also influences the ease of person-to-person transmission at relative humidities of 30 to 50 % 23. Also,17 detected SARS-CoV-2 RNA in different materials, highlighting metal as the one with the highest amount at temperature (20 - 25 °C) and humidity (30 - 86 %) sampling.
Recent studies report that at concentrations of 1-6 ppm for times of 55-60 min SARS-CoV-2 is inactivated up to 90% in controlled environments with a relative humidity of 60-80%. In the present study, the virus was eliminated at shorter exposure times, lower ozone concentrations and lower relative humidity ratios, demonstrating the feasibility of using this technology in enclosed spaces such as laboratories, clinics, and classrooms for inactivation of SARS-CoV-2 on surfaces.26 Likewise, the ozone concentrations used in the present study do not represent a health risk, since at concentrations lower than 0.2 ppm ozone has not been shown to generate pulmonary effects in humans, as well as its rapid decomposition in the environment, between 20-30 minutes under normal temperature and humidity conditions, which, again, does not represent an exposure problem.32 Likewise, as an advantage, the fast disinfection times in this type of space and surfaces are presented, thus demonstrating the feasibility of using this technology in closed spaces, with an influx of people to reduce exposure to SARS-CoV-2.
It is known that disinfection with 0.1% sodium hypochlorite and/or 62-71% ethanol in an exposure time of 1 minute can eliminate 99% of this virus on non-porous surfaces,6,24 due to this, the samples taken from the metal surface of the biosafety hood were negative since the frequency of cleaning is constant. Regard the sanitization with sodium hypochlorite, the lack of regulation of chlorine used as a disinfectant has developed adverse effects on the environment and public health. Albert et al., show the amount of inactivation of the virus, 82 to 91.5 %, with 0.75 ppm dissolved ozone. Therefore, the safety and viability for the sanitization of surfaces made up of different materials. Hence, the use of ozone with the absence of fumes and in low concentrations, the possibilities of showing adverse effects must be short. In addition, the use of ozone for disinfection and inactivation of SARS CoV - 2 has been demonstrated to be an option with low risk, cost, high efficiency, and volume of action for its use in workplaces.
Despite the above, direct ozone exposure treatment eliminated SARS-CoV-2 virus RNA on the previously sampled surfaces. Likewise, no bacteria grew on the previously sampled agar boxes. This has been seen in both personal protective equipment25 and in vitro assays.26 Because the mechanism of action of ozone against enveloped viruses, such as SARS-CoV-2, begins with the generated ozone rapidly reacting with the surface capsid of membranes, disabling both membrane receptors and protein functional groups; it also induces peroxidation of phospholipids, also the production of reactive oxygen species and thus damages to the DNA or RNA of these viruses.27
Although different exposure times have been elucidated that can lead to effectiveness, it has been observed that temperature and humidity play an important role in the survival of the virus and its inactivation by ozone. As mentioned by25 where note that by making modifications to these conditions, exposure times can be reduced from 4 h to 5 min. Evidence has shown that ozone is capable of breaking the cell membrane or protoplasm, making it impossible to activate bacterial, viral, and protozoan cells, eliminating up to 99% of bacteria and viruses at 10 mg/L in 10 minutes. It attacks mainly unsaturated fatty acids, lipid fatty acids, glycoproteins, glycolipids, amino acids, and sulfhydryl groups of certain enzymes.
DNA and RNA are not resistant to ozone, different studies have shown that ozone can destroy pathogenic and non-pathogenic microorganisms such as viruses, bacteria, fungi, spores, protozoa, nematodes (helminth eggs), and algae.31 Ozone causes oxidation or ozonolysis of certain amino acid residues, e.g. tryptophan, tyrosine, and cysteine. As a result of this attack, protein molecules change their usual folding and binding capacity and become denatured, inhibiting their biological activity.29 Our results demonstrate that, as previously reported, ozone, in addition to inactivating SARS-CoV-2, is capable of reducing and eliminating contamination by other microorganisms, thus providing safe spaces for people and decreasing the risk of infection by various agents.
Although we have not evaluated the presence of the virus elsewhere in the facility, the indication of an insufficient frequent disinfection regime in these laboratories could be a reflection of other high-contact surfaces in the building. As we could observe with this study if there is RNA in a general entrance, it may be in other more crowded places, as well as in the same environment. Therefore, hygiene education campaigns should be reinforced among the occupants of the building to avoid contamination and eventual infection.
Our study highlights the importance of evaluating the presence of the virus, not only in laboratories but also on surfaces where people work daily. To adopt new disinfection measures and adhere to basic personal protective equipment to reduce the presence of SARS-CoV-2 in the workplace, it is also necessary to adopt ozone sterilization as a method of disinfection in addition to synergism with other methodologies to achieve greater effectiveness and lower probability of infection.
None.
Author declares that there is no conflict of interest.

©2022 Lopez-Mendoza, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.