Journal of
eISSN: 2373-437X


Research Article Volume 10 Issue 6
Taras Shevchenko National University of Kyiv, Kyiv, Ukraine
Correspondence: Ganna Tolstanova, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine, Tel +380731584360
Received: November 02, 2022 | Published: November 21, 2022
Citation: Serhiichuk T, Aleksandrova I, Korbush M, et al. Nigella sativa oil of «diana» sort in the therapy of antibiotics simulated dysbiosis. J Microbiol Exp. 2022;10(6):195-200. DOI: 10.15406/jmen.2022.10.00373
Dysbiotic disorders of the gastrointestinal tract (GIT) are one of the urgent problems today. There are a number of directions and means aimed at restoring the normobiota of the GIT, comprising of probiotics. An alternative to them can be probiotics and nutraceuticals, which are used in cases when the application of live strains is contraindicated. The purpose was to assess the possibility of using Nigella sativa oil to eliminate antibiotic-associated dysbiosis disorders.
Methods: Dysbiosis was modeled by administering of ampicillin (75 mg/kg) and metronidazole (50 mg/kg) to non-linear male rats (170-200g, n=49) for 7 days. N. sativa «Diana» variety oil (200 mg/kg) was administered to the animals after antibiotics withdrawal. In comparison were used oil: N. sativa «Ancient Traditions», Ukraine (200 mg/kg); N. sativa «Messenger's Speech», Egypt (200 mg/kg), home-pressed sunflower oil (200 mg/kg); prebiotic «Healthy Tract Prebiotic» (30 mg/kg). All preparations were administered per os for 7 days. The quantitative composition of the microbiota was determined by plating 10-fold dilutions of fecal biopsies on elective media, expressed in lg (m+M) CFU/g.
Results: The study has demonstrated a significant decrease in Lactobacillus and lactose-positive E. coli by 2 orders as well after antibiotic modeled dysbiosis. On the contrary, there was a significant increase in the number of lactose-negative E. coli by 4 orders and conditionally pathogenic enterobacteria (CPE) by 3 orders. Administration of N. sativa «Ancient Traditions» and «Messenger's Speech» to animals contributed to the complete restoration of the disturbed microbiota. The use of prebiotic partially contributed to the restoration of lactobacilli and lactose-positive E. coli titers, but didn`t eliminate the changes in lactose-negative E. coli and CPE. N. sativa«Ancient traditions» and sunflower oil did not have a positive effect on the restoration of the GIT microbiota at all.
Conclusion: The use of N. sativa «Diana» variety oil for 7 days contributes to the complete restoration of the normobiota disturbed by the administration of antibiotics.
Keywords: microbiota, dysbiosis, antibiotics, nutraceuticals, prebiotics
The microbiota of the human body is the foundation for maintaining human homeostasis.1,2 Changes in the quantitative and qualitative composition of the microbiota inevitably lead to the emergence of pathological conditions.3 Many reasons can lead to a violation of the normobiocenosis of the digestive tract: stressful situations, a change in the type of diet, environmental factors, various diseases, and the therapy of these diseases.4-10 There are several ways to restore the normobiota of the gastrointestinal tract, due to the presence of vital probiotics.8,11-17 But in the presence of such conditions as immunodeficiencies, disruption of the epithelial barrier, etc., the use of living cells of microorganisms is not recommended.9,18 And in this case, prebiotics and nutraceuticals can become promising.
One of the ways to find new highly effective and safe therapeutic agents is to switch on to the conventional folk medicine. The unique therapeutic properties of black cumin have been known for more than 3,000 years.19
To date, the biological properties of Nigella sativa have been thoroughly studied and it has been scientifically proven that it has a wide range of effects, like diuretic, hypotensive, antidiabetic, antitumor, and immunomodulatory, analgesic, antimicrobial, anthelmintic, analgesic and anti-inflammatory, antispasmodic, bronchiolitis, gastroprotective, hepatoprotective, kidney protective and antioxidant properties. N. sativa seeds are widely used in the treatment of various diseases such as bronchitis, asthma, rheumatism, skin diseases, digestive therapy as an anti-diarrheal agent, appetite stimulant, to fight against parasitic infections and cancer, to support the immune system, and many other pathologies.20-25
The therapeutic properties of black cumin are associated with such substances as thymoquinone (30%-48%), thymohydroquinone, dithymoquinone, p-cymene (7%-15%), carvacrol (6%-12%), 4-terpineol (2 %-7%), t-anethol (1%-4%), sesquiterpene longifolene (1%-8%) α-pinene and thymol, etc. Black seeds also contain some other compounds in trace amounts. Seeds contain two different types of alkaloids; i.e. isoquinoline alkaloids e.g. nigellicimine and nigellicimine-N-oxide, and pyrazole alkaloids or indazole ring bearing alkaloids which include nigellidine and nigellicine. Moreover, N. sativa seeds also contain alpha-hederin, a water soluble pentacyclic triterpene and saponin, a potential anticancer agent.19,26 Different sorts of black cumin contain many other substances,27 the presence of which largely depends on the variety and area of cultivation of the plant.28
N. sativa is an aboriginal plant of Southern Europe, Northern Africa, and Southwest Asia and is cultivated in many countries of the world, including Mediterranean region of the Middle East, Southern Europe, India, Pakistan, Syria, Turkey, and Saudi Arabia. Nowadays, black caraway is also cultivated in Ukraine as a decorative and spicy plant. In 2011, Anatoly Pedko obtained a new «Diana» sort in Cherkasy region.29 Since there are reports that the therapeutic properties of N. sativa may differ depending on the region of cultivation,30-33 and also because of the limitations of research on the use of various types of its oil in the therapy of dysbiosis, we considered it appropriate to evaluate the possibility of using N. sativa oil of the «Diana» sort in Ukraine for the elimination of antibiotic-associated dysbiotic violations.
The object of the study was the fecal microbiota of the colon of non-linear male rats (n=49, weighing 170-200 g).
Drugs used in the work:
Experiment design
Dysbiosis was modeled by administering to non-linear male rats (weight 170-200 g, n=42) a complex of 2 antibiotics: ampicillin (at a dose of 75 mg/kg) and metronidazole (at a dose of 50 mg/kg) for 7 days(Ermolenko et al., 2013). After the antibiotics administration was discontinued, Nigella sativa oil of the “Diana” sort (200 mg/kg) was administered to the animals. The following drugs were used as comparison drugs: N. sativa oil «Ancient traditions» (200 mg/kg); N. sativa oil “Messenger's Speech”, El Hawag, (200 mg/kg), home-pressed sunflower oil (200 mg/kg); prebiotic «Healthy Tract Prebiotic» (30 mg/kg). All drugs were administered per os for 7 days (Figure 1).
Cultivation of 10-fold dilutions was carried out on differential diagnostic media with selective properties: Endo, Simmons Citrate Agar, 5% Blood Agar, Mannitol-SaltAgar, Bifidobacterium Agar, Lactobacillus MRS Agar (HiMedia Laboratories Pvt. Ltd., India) to determine the quantitative and qualitative composition of individual indicative representatives of the microbiota. Further identification of the isolated microorganisms was carried out according to morphological,tinctorial, physiological and metabolic indicators (reaction to plasma coagulation, DNAse activity, production of lysozyme, phosphatase, sensitivity to novobiocin to separate S. aureus from S. saprophyticus; oxidase test, carbohydrate fermentation tests, Voges-Proskauer reaction, motility test, hydrogen sulfide formation to separate lactose-negative E. coli from opportunistic enterobacteria). The results are presented as M±mlg CFU/g.
Statistical processing of the results were carried out using Excel and STATISTICA. The arithmetic mean and error of the mean were calculated. The significance of the difference between the control and experimental groups was assessed using the Student's t-test for independent samples.
Changes in the composition of the microbiota during ampicillin/metronidazole simulated dysbiosis were recorded on the first and eighth days (respectively, the 8th and 16th (see Figure 1-4) days of the experiment) after the withdrawal of antibiotics (Table 1).
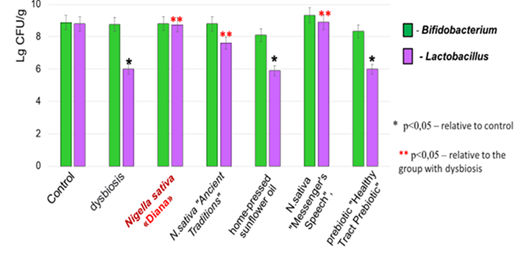
Figure 2 Quantitative changes in obligate sucrolytic bacteria during antibiotic-simulated dysbiosis and its correction.
Microorganism |
Control (n=49) |
A day after the withdrawal of the combination ampicillin with metronidazole |
|
1th day (n=42) |
8th day (n=7) |
||
Bifidobacterium sp. |
8,86 ± 0,24 |
8,71 ± 0,64 |
8,76 ± 0,9 |
Lactobacillus sp. |
8,79 ± 0,16 |
6,32 ± 0,15* |
6,01 ± 0,81* |
E.сoli (lactose fermenting) |
5,21 ± 0,27 |
1,31 ± 0,91 |
3,41 ± 0,52* |
E.сoli (lactose non-fermenting ) |
1,72 ± 0,85 |
0* |
6,52 ± 0,20* |
Conditionally pathogenic enterobacteria |
1,08 ± 0,44 |
0* |
4,10 ± 0,47* |
Staphylococcus aureus (mannitol-positive) |
3,41 ± 0,07 |
3,40 ± 1,51 |
6,26 ± 0,84* |
Staphylococcus sp. (mannitol-negative) |
1,21 ± 0,31 |
0* |
4,68 ± 0,61* |
Table 1 Dynamics of changes in the main representatives of the fecal biotope(М±mlg CFU/g) after the administration of combination ampicillin with metronidazole
*р<0,05 – compared to Control
It is shown that the quantitative indicators of representatives of the genus Bifidobacterium remained within the control values throughout the experiment. The number of representatives of the genus Lactobacillus decreased by 2 orders of magnitude compared to the control on the first day after the withdrawal of antibiotics and did not recover within 7 days.
Quantitative indicators of lactose-positive Escherichia coli decreased on the first day after the withdrawal of antibiotics from lg 5.21 ± 0.27 CFU/g to lg 1.31 ± 0.91 CFU/g. Seven days after antibiotic administration was discontinued, their number increased slightly (up to lg 3.41 ± 0.52 CFU/g), but these indicators were lower than the control by two orders.
Lactose-negative Escherichia coli completely disappeared from the fecal biotope immediately after the withdrawal of antibiotics, but after seven days their growth was noted by five orders compared to the control group.
A similar trend was observed for opportunistic enterobacteria. Immediately after the withdrawal of antibiotics, they were not cultivated from the fecal biotope, but after seven days their number increased to lg 4.10 ± 0.47 CFU/g, which are 3 orders higher than the control values (lg 1.08 ± 0.44 CFU/g).
Mannitol-positive Staphylococcus were resistant to the introduction of ampicillin with metronidazole and their number remained unchanged immediately after the withdrawal of antibiotics. But seven days after the withdrawal of antibiotics, an increase in their number was observed from lg 3.41 ± 0.47 CFU/g.
Mannitol-negative Staphylococcus disappeared from the fecal biotope immediately after the discontinuation of antibiotics. Seven days after simulated dysbiosis, their number increased from lg 1.21 ± 0.31 CFU/g (control) to lg 4.68 ± 0.61 CFU/g.
Thus, seven days after the discontinuation of ampicillin with metronidazole, a significant decrease in the number of anaerobic sucrolytic bacteria of the genus Lactobacillus and lactose-positive E. coli and, conversely, a significant increase in lactose-negative E. coli, opportunistic enterobacteria and representatives of the genus Staphylococcus (both mannitol-positive and mannitol-negative forms) were noted.
In further studies, the eighth day after the introduction of antibiotics, which is designated as «dysbiosis», was chosen for comparison.
During restorative therapy with the use of oils of different manufacturers and prebiotics, it was shown that when N. sativa oil of the «Diana» sort was administrated, the quantitative indicators of genus Lactobacillus representatives were restored to control values (lg 8.73 ± 0.29 CFU/g). The same results (lg 8 .83 ± 0.21 CFU/g) were also observed when N. sativa«Messenger's Speech» oil was administered. When using N. sativa«Ancient Traditions» oil, there was also a tendency to restore quantitative indicators of Lactobacillus (lg 7.72±0.56 CFU/g) in comparison with the «dysbiosis» group (lg 6.01 + 0.81 CFU/g) but these indicators were lower than the control level by one order (control lg 8.79 ± 0.16 CFU/g). The introduction of sunflower oil and the prebiotic «Healthy Tract Prebiotic» did not lead to the restoration of the number of Lactobacillus (Table 2).
Тhe research group |
Bifidobacterium sp. |
Lactobacillus sp. |
Control (n=49) |
8,86 ± 0,24 |
8,79 ± 0,16 |
Dysbiosis (n=7) |
8,76 ± 0,15 |
6,01 ± 0,81* |
N.sativa«Diana» (n=7) |
8,87 ± 0,18 |
8,73 ± 0,29** |
N.sativa«Ancient Traditions» (n=7) |
8,71 ± 0,15 |
7,72 ±0,56** |
N.sativa«Messenger’s Speech» (n=7) |
9,15 ± 0,41 |
8,83 ± 0,21** |
home-pressed sunflower oil (n=7) |
8,02 ± 0,14 |
5,86 ± 0,35* |
prebiotic «Healthy TractPrebiotic»(n=7) |
8,13 ± 0,29 |
5,93 ± 0,18* |
Table 2 Quantitative changes (М±mlg CFU/g) in obligate sucrolytic bacteria during antibiotic-simulated dysbiosisand its correction
*р<0,05 – compared to Control
**р<0,05 – compared to dysbiosis group
One of the important indicators of dysbiosis development is changes in the composition of such gram-negative representatives of the Enterobacteriaceae family as lactose-fermenting and lactose-non-fermenting E. coli and other conditionally pathogenic genera (Table 3). It was demonstrated that when N. sativa oil of «Diana» and «Messenger's Speech» sorts was introduced, the number of lactose-positive E. coli was restored to normal values. The introduction of sunflower oil and prebiotics did not contribute to the recovery of this indicator.
Тhe research group |
E.сoli (lactose fermenting) |
E.сoli (lactose non-fermenting ) |
Conditionally pathogenic enterobacteria |
Control (n=49) |
5,21 ± 0,27 |
1,72 ± 0,85 |
1,08 ± 0,44 |
Dysbiosis (n=7) |
3,41 ± 0,52* |
6,52 ± 0,20* |
4,10 ± 0,47* |
N.sativa«Diana» (n=7) |
4,86 ± 0,91 |
1,15 ± 0,32** |
0** |
N.sativa«Ancient Traditions» (n=7) |
4,15 ± 0,86 |
5,53 ± 0,16 |
4,18 ± 0,19 |
N.sativa«Messenger's Speech» (n=7) |
5,03 ± 0,32 |
1,52 ± 0,41** |
0** |
home-pressed sunflower oil (n=7) |
1,17 ± 0,37* |
5,83 ± 0,19 |
4,61 ± 0,81 |
prebiotic «Healthy Tract Prebiotic» (n=7) |
2,81 ± 0,15* |
4,73 ± 0,18 |
3,96 ± 0,81 |
Table 3 Quantitative changes (М±mlg CFU/g) of enterobacteria in antibiotic-modeled dysbiosis and its correction
*р<0,05 – compared to Control.
**р<0,05 – compared to dysbiosis group.
The number of lactose-negative E. coli under modeled dysbiosis increased from lg 1.72 ± 0.85 CFU/g (control) to lg 6.52 ± 0.20 CFU/g. When using N. sativa oil of the «Diana» and «Messenger's Speech» sorts, these indicators were restored to the limits of the control values. Quantitative indicators of lactose-negative E. coli with the introduction of N. sativa oil «Ancient traditions», sunflower oil, and prebiotic «Healthy Tract Prebiotic» were lg 5.53 ± 0.16 CFU/g, lg 5.83 ± 0.19 CFU/g, lg 4.73 ± 0.18 CFU/g, respectively, which is only 1-1.5 orders lower than the similar indicator in dysbiosis.
The use of N. sativa oil of the «Diana» and «Messenger's Speech» sorts also had a positive effect on conditionally pathogenic enterobacteria, which disappeared from the fecal biopsy after these drugs were administered, while the use of N. sativa oil «Ancient Traditions», sunflower oil and prebiotic «Healthy Tract Prebiotic» did not affect conditionally pathogenic enterobacteria at all.
Antibiotics withdrawal contributed to the increase in the number of both mannitol-positive (Staphylococcus aureus) and mannitol-negative (Staphylococcus epidermidis, Staphylococcus saprophyticus) Staphylococcus by 3-4 orders. When N. sativa oil of the «Diana» and «Messenger's Speech» sorts was administered to animals with simulated dysbiosis, a decrease in the number of both groups of the genus Staphylococcus was noted, even lower than in control animals. Administration of N. sativa oil «Ancient Traditions», sunflower oil, and prebiotic «Healthy Tract Prebiotic» practically did not affect the quantitative levels of staphylococci (Table 4).
Тhe research group |
Staphylococcus aureus (mannitol-positive) |
Staphylococcus sp. (mannitol-negative) |
Control (n=49) |
3,41 ± 0,07 |
1,21 ± 0,31 |
Dysbiosis (n=7) |
6,26 ± 0,84* |
4,68 ± 0,61* |
N.sativa«Diana» (n=7) |
1,51 ± 0,32** |
1,02 ± 0,35** |
N.sativa«Ancient Traditions» (n=7) |
5,34 ± 0,56 |
2,79 ±0,12** |
N.sativa«Messenger's Speech» (n=7) |
1,03 ± 0,08** |
0** |
home-pressed sunflower oil (n=7) |
6,02 ± 0,81 |
3,21 ± 0,47* |
prebiotic «Healthy Tract Prebiotic» (n=7) |
5,89 ± 0,19 |
4,51 ± 0,37* |
Table 4 Quantitative changes (М±mlg CFU/g) of staphylococci in antibiotic-modeled dysbiosis and its correction
*р<0,05 – compared to Control.
**р<0,05 – compared to dysbiosis group.
It was shown that the use of Nigella sativa oil of the «Diana» sort for 7 days contributes to the complete restoration of thenormobiota disturbed by the administration of ampicillin and metronidazole.
Earlier studies have shown that dysbiosis, occurs as a result of the use of antibiotics, has a long-term nature, is not capable of disappearing on its own, and requires treatment.34-36 Since there are conditions in which the use of probiotics is not recommended, we assumed that in such cases it may be appropriate to use products with prebiotic and nutraceutical effects. Black cumin can have a double effect on both the microbiota and intestinal epitheliocytes. There are reports of its bactericidal properties37,38and its ability to influence the normalization of the digestive tract,38,39 but we have not come across reports of its use to restore antibiotic-associated dysbiosis.
In our experiments to restore the microbiota disturbed by the complex with the introduction of antibiotics (ampicillin and metronidazole), we used oil obtained by cold pressing from the seeds of Nigella sativa grown in Ukraine and compared it with two brands of black cumin oil purchased in pharmacy chains, a dietary supplement with probiotic properties «Healthy Tract Prebiotic» which component is black cumin seed extract and home-pressed sunflower oil.
It should be noted that sunflower oil was used as a neutral control and it was shown that it had no positive effect on the recovery of intestinal microbiota. In scientific sources, there are no reports of its use in dysbacteriosis.
The multicomponent prebiotic «Healthy Tract Prebiotic» from Irwin Naturals, which has a high rating of positive feedback among consumers40 in our studies did not reveal a positive dynamic in the ability to restore the antibiotic-disrupted normobiota. We explain the low effectiveness of this prebiotic by our non-compliance with the recommendations regarding the timing of its administration. In the instructions for use, the duration of its use should be 4 weeks, and in our studies, the period of its administration was 1 day.
Black cumin oil of the «Ancient Traditions» brand is produced in Ukraine by cold pressing, but without indicating the variety and origin of the seed as the basis of its component. In our study, this brand of oil had no effect on the recovery of the normobiota. We attribute the lack of effect on the microbiota of this cumin oil to its low quality, as it was more similar to sunflower oil in terms of color, smell, and taste. We did not determine the chemical composition of the oil.
«Messenger's Speech» – black cumin oil of one of the most famous manufacturer brands, made in Egypt from seeds from Ethiopia, proved to be highly effective in restoring antibiotic-associated dysbiosis. Similar results were obtained when using «Diana» black cumin oil. When using these oils, rapid recovery of the quantitative indicators of the genus Bifidobacterium and lactose-fermenting E. coli was noted, as well as the elimination of opportunistic enterobacteria and staphylococci from the intestinal biotope.41-43
Thus, Nigella sativa oil of the Diana sort can be recommended for use in restoring the normobiota disturbed by antibiotic therapy.
The use of Nigella sativa oil of the «Diana» sort grown in Ukraine for 7 days contributes to the complete restoration of the normobiota disturbed by the administration of ampicillin and metronidazole.
The research project was fully sponsored by the Ministry of Education and Science of Ukraine with grant number (grants #11BF036-01, #19BF036-01). The cold-pressed oil of Nigella sativa sort «Diana» from seeds grown in Cherkasy region (Ukraine) has been sponsored by UkrEXPOPROCESS LLC (Kyiv, Ukraine).
The authors declare that there is no conflict of interest.

©2022 Serhiichuk, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.