Journal of
eISSN: 2373-437X


Research Article Volume 10 Issue 6
1Professor and Director of Research at UnirG-Campus de Paraíso do Tocantins, Brazil
2Professor at Federal University of Goiás (UFG), Head of Laboratory of Invertebrate Control (LCI), Department of Genetics, Institute of Biological Sciences (ICB) of UFG, Brazil
3Professor at Federal University of Goiás (UFG), Goiânia, Goiás, Brazil. Associate Researcher at LCI/ICB/UFG (Project number PI02975-2018)
Correspondence: Professor Fernando de Freitas Fernandes. Review Division, Editorial Center of the Federal University of Goiás (Cegraf UFG), Avenida Esperança s/n, Campus Samambaia, Goiânia, GO, 74690-900, Brazil. , Tel +5562985803509
Received: November 20, 2022 | Published: December 12, 2022
Citation: D´Alessandro WB, Leles RN, Fernandes FF. Monitoring of evolution of resistance to commercial acaricidal products in Rhipicephalus sanguineus sensu lato (Latreille, 1806) (Acari: Ixodidae) from Goiânia, Goiás State, Brazil. J Microbiol Exp. 2022;10(6):216-222. DOI: 10.15406/jmen.2022.10.00376
The Brown dog tick Rhipicephalus sanguineus is the most reported and geographically widely spread tick in the world, presenting a great medical and veterinary importance, mainly because the ability to transmit various diseases to its hosts, causing great harm to pets, but also risks to public health, due to the transmission of emerging pathogens to humans. In order to monitor the resistance or susceptibility of R. sanguineus to acaricides, and contribute to integrated control measures for this vector, the activity of 14 commercial acaricidal products on larvae from the city of Goiânia, Goiás, Brazil were studied. Although these products are already sold as acaricides, their cost-benefit efficiency has been questioned by dog breeders and kennel owners. R. sanguineus larvae were obtained from engorged females collected in naturally infested urban environments. The susceptibility of the larvae was evaluated by the larval packet test (lpt) method. Bioassays were carried out in quadruplicate, at 27 ± 1oC, RH ≥ 80% and photoperiod of 12 hours. About 50 larvae, from 14 to 21 days of age, were conditioned in filter paper envelopes, impregnated with different concentrations of acaricide products, obtained from dilution of stock solutions. The control group used the same amount of larvae, submitted to envelopes treated only with distilled water. A status of resistance of R. sanguineus larvae to acaricide formulations was evidenced for Cypermethrin, Cypermethrin + Piperoline Butoxide, Deltamethrin, Permethrin (after 24h of exposure), in the dosages recommended by the manufacturing laboratories, as they promoted a mean mortality of only 58.36%, 71.36%, 48.7%, and 64.5% of the submitted larvae, respectively, Amitraz providedof only 78.8 and 88.00% (After 24 and 48 hours of exposure to the acaricide, respectively), characterizing a status of possible development of resistance to this acaricide. The other evaluated products (Dichlorvos + Cypermethrin, Chlorfenvinphos + Dichlorvos + Alkyl + Xylol, Chlorpyrifos + Cypermethrin hi-cis, Fipronil, Chlorpyrifos, Cypermethrin + Chlorpyrifos + Citronellal, and Trichlorfon + Coumaphos + Cyfluthrin; and Diazinon) showed higherindices, configuring the susceptibility status to these acaricide formulations. No significant mortality was found in the control groups. The ideal concentrations to kill R. sanguineus larvae (CL99) referring to products that showed low acaricidal activity were statically calculated and are presented in this study. The present work, confronted with previous studies, ratified the real evolution of resistance to synthetic chemical acaricides in local populations of R. sanguineus.
Keywords: ixodidae, Rhipicephalus sanguineus, tick control, acaricide resistance, emerging tick-borne diseases in humans
Rhipicephalus sanguineus (Latreille,1806) (Acari: Ixodidae) is a three-host tick, which in its tropical and temperate lineages is considered one of the ixodid species with the widest geographic distribution, being present in American, European, African and Asian countries, e.g. Brazil, Uruguay, Argentina, Chile, Mexico, United States, Portugal, Spain, Italy, France, Switzerland, Mozambique, India, among others countries.1-4 Despite parasitizing mainly domestic dogs, being the most prevalent tick on these hosts in urbanized areas of Brazil and other American countries, this ixodid can, however, parasitize other mammals, birds and reptiles, which partly explains its wide dispersion.5-7
Different strains of R. sanguineus complex are involved in the transmission of various pathogens to their hosts, such as Babesia canis, B. gibsoni, B. caballi, Ehrlichia canis, Hepatozoon canis, Haemobartonella canis, Theileria equi, and Rickettsia spp. Living with domestic dogs infested by this tick results in occasional parasitism in humans, mainly due to its immature forms, with an increase in the number of reports of this parasitism, as well as in the diagnosis of emerging human pathogens in this tick, including Rickettsia rickettsii, R, conorii, R. parkeriand, R. amblyommatis, in addition to Candidatus Rickettsia andeanae, etiological agents of diseases of the etiological group of spotted fever in several parts of the world.2,8-18
A large sum of resources has been employed in the use of chemical acaricides as the main way to combat ticks, however, the indiscriminate use of these compounds in tick control has led to a large increase in selection pressure for acaricide resistance. Development of resistance in R. sanguineus to arsenical acaricides,19 organophosphates, carbamates20 and organochlorines21 was observed in the 70's. In Brazil, the first works on the chemical control of this species only appeared from the mid-1990s, as well as the first report of the development of acaricide-resistant populations of R. sanguineus s. l. (tropical lineage) in Brazil, carried out by Fernandes FF and his team of student researchers.6,22-25 More recently, the development of tolerance or resistance to different acaricide bases in different populations of Rhipicephalus sanguineus has been reported in other locations around the world.4,26-29
The accomplishment of regional studies to monitoring the susceptibility or resistance of R. sanguineus to chemical acaricides is of fundamental importance to guide local communities in choosing more efficient acaricide products, with a view to the health of domestic dogs, the reduction of the environmental impact with the rational use of these toxic products, and the decrease in the occurrence of cases of emerging tick-borne diseases in humans.
Therefore, the aim of the present work was to investigate the activity of 14 formulations of chemical acaricides available on the market, developed for the control of ticks and other ectoparasites, at different concentrations, on larvae of R. sanguineus, for promoting more efficient and sustainable integrated control measures to this vector.
Obtaining of R. sanguineus larvae
Engorged females (teleogynes) of this species were collected in environments frequented by dogs naturally infested, in several neighborhoods of Goiânia, in Goiás state, Brazil. These were stored in glass flasks with proper cover and then sent to the laboratory, where they were washed in distilled water, dried on paper towels. With aid of a stereoscope, they were taxonomically and only teleogynes in perfect anatomical conditions were selected for the study. For the accomplishment of the oviposition, the teleogynes were adhered dorsally with doubled sided tape on slides, inversely disposed on the base of a dish Petri. Later on they were conditioned in an incubator of the type B.O.D., acclimatized to 27±1oC, RH ≥ 80% and photoperiod of 12 hours. Daily ovipositions were collected and put together in a single polyethylene tube (3 x 9cm) with a screw-on cover, constituting an egg pool. After the larvae hatched, each polyethylene tube was then sealed with adhesive tape to prevent larvae escape.6
Preparation of tested solutions
Fourteen different formulations (Table 1), usually marketed to combat ticks and insect parasites and vectors, were evaluated (For ethical reasons, it was decided not to mention the manufacturing laboratories and commercial names of the tested products, only the bioactive compounds in their formulations). The bioassays were performed in quadruplicate. For each replicate, new stock solutions were prepared at different concentrations according to the acaricide to be evaluated. From the stock solution, Solutions with lower concentrations were obtained, including those recommended by the product manufacturers, through a progressive dilutions series with distilled water.30,31
|
Treatment |
Chemical Group |
Median Mortality (%) |
|
Dichlorvos + Cypermethrin |
Organophosphorus + synthetic Pyrethroid |
96.00 a |
|
Chlorfenvinphos + Dichlorvos + Alkyl + Xylol |
Organophosphorus + surfactant + solvent |
100.00 a |
|
Chlorpyrifos + Cypermethrin hi-cis |
Organophosphorus + synthetic Pyrethroid |
100.00 a |
|
Cypermethrin |
synthetic Pyrethroid |
58.36 c |
|
Amitraz (after 24h)µ |
Formamidine |
77.80 b |
|
Amitraz (after 48h)σ |
Formamidine |
88.00 a |
|
Fipronil |
Pyrazole |
100.00 a |
|
Cypermethrin + Piperonyl butoxide+ citronellal |
Pyrethroid + synergistic compounds |
71.63 b |
|
Chlorpyrifos |
Organophosphorus |
100.00 a |
|
Cypermethrin + Chlorpyrifos + Citronellal |
Pyrethroid + Organophosphorus + synergistic compound |
100.00 a |
|
Deltamethrin |
Synthetic Pyrethroid |
48.7 c |
|
Triclorfon + Coumaphos + Cyfluthrin |
organophosphates + Pyrethroid |
100.00 a |
|
Diazinon |
Organophosphorus |
100.00 a |
|
Permethrin |
Synthetic Pyrethroid |
64.50 b |
|
Chlorpyrifos + Amitraz (after 24h)µ |
Organophosphorus + Formamidine |
100.00 a |
|
Chlorpyrifos + Amitraz (after 48h)σ |
Organophosphorus + Formamidine |
100.00 a |
Table 1 Toxicological effect of acaricidal products on Rhipicephalus sanguineus larvae, according to their respective dosages recommended by the manufacturing laboratories, in Goiânia, Goiás State, Brazil
*For ethical reasons, it was decided not to mention the manufacturing laboratories and commercial names of the tested products, only the bioactive compounds in their formulations.
Exposure time to the acaricide, for 24hµ and 48h.σ
Equal letters in the same column indicate that there is no significant difference in mortality promoted by the products, in the doses recommended by their manufacturing laboratories. (ANOVA followed by the Scott-Knott test, α = 5%).
Bioassays for evaluating larval sensitivity
The impregnated papers methodology, also known as larval packet test (lpt), was used. This method was adapted by Fernandes and Freitas30 from previously described methods, mainly those of FAO,32,33 Fernandes6,34 and Fernandes et al.35,36 Adaptations of the FAO method were carried out with the aim of increasing practicality and reducing costs, but without loss of efficiency, having been tested and improved, with satisfactory results in the evaluation of the acaricidal activity of chemical and botanical substances on different species of Ixodidae, e.g., R. sanguineus, R. microplus and Amblyomma cajennense. Further details of the methodology can be found in Fernandes and Freitas.30,31
The bioassays were carried out in a biological chamber, acclimatized at 27±1oC and RH ≥ 80%, photoperiod of 12 h, and built especially for studies with acaricidal substances. Only larvae from egg pool tubes with the highest hatchability rates (> 90%) were used in the bioassays. To contain and expose the larvae to the tested solutions, filter paper envelopes (≈ 327 cm2) containing micropores were used to provide ventilation inside. The internal surfaces of each envelope were impregnated with 2 mL of each tested solution, evenly distributed using a pipette. The envelopes were left to rest for 6 h, for the crystals formed by the acaricides to adhere to the filter paper. The test group was submitted to envelopes impregnated with acaricide solutions. The bioassays were performed in quadruplicate, on different dates. In each replica four envelopes were prepared for each tested concentration. About 50 or more larvae were nimbly lifted from the white paper fixed on the bench, with a brush moistened with the test solution (test group) or with distilled water (control group) and placed at the bottom of each envelope. The envelopes were immediately sealed, folding their opening, a 1 cm border and sealing it with the aid of a metallic fastener. The envelopes were then hung on supports, so that they were suspended in the air, without contact with any surface, in order to avoid loss of solution or contamination. Humidifiers were arranged under the envelopes on the benches to maintain adequate climatization of the biological chamber.30
The envelopes were opened and inspected by the stereoscope after 24 and 48 hours of exhibition, for the registration of alive and dead larvae and possible observed toxicological effects. To make possible the comparison of the results with other authors studies, the mean percentage mortality was calculated considering also killed the larvae without capacity of locomotion. Replicates in which control mortality exceeded 5% discarded and repeated.32,33
Recommended guidelines for diagnosing acaricide resistance status
The efficiency of the treatments for diagnosing the status of resistance and/or susceptibility to the acaricidal product considered the criteria recommended by the World Health Organization (WHO).37,38 Acaricidal products that promoted medium mortality rates of larvae <80%, at the concentration recommended by the manufacturer's laboratory, were considered ineffective, characterizing the resistance status. Acaricides that caused mortality ≥80% characterized a susceptibility status. The criteria determined by the Brazilian Ministry of Agriculture, Livestock and Supply (2015)39 were also considered, which determines at 95% the minimum level of efficacy necessary for the approval of the registration and commercialization of an acaricidal product for veterinary use in the country.
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) followed by the Scott-Knott test, using the Assistat® software v. 7.4., to verify significant differences between the mortalities determined by the acaricides in their respective dosages recommended by the manufacturer. Lethal concentrations (LCs), especially CL50 and CL99 with their respective 95% confidence intervals (95% CI) and their coefficients of determination (R2) were determined for the products that proved to be ineffective, interpolating the mortality rates provided by the different concentrations tested, through Probit analysis, using the System for Statistical and Genetic Analysis (SAEG® software v. 9.0).31
R. sanguineus larvae responded heterogeneously to the fourteen acaricidal products analyzed (ANOVA: F 14., 30 = 9.23; p<0.01). The analyzed products Cypermethrin, Cypermethrin + Piperoline Butoxide, Deltamethrin, Permethrin, and Amitraz (24 h of exposure) promoted mortality lower than 80% (Table 1), characterizing the resistance status of larvae to these chemical formulations, according to WHO guidelines (Table 1).
It is relevant to point out that the action of the product based on Amitraz, even though it did not characterize the resistance status of larvae (WHO),38 after 48h of exposure, determined a scenario of possible development of resistance, as it promoted an average mortality rate of only 88%, which, according to the guidelines of the Brazilian Ministry of Agriculture, Livestock and Supply (2015)39 would prevent the recommendation of this product to control this tick.
The other evaluated acaricide formulations – Dichlorvos + Cypermethrin; Chlorfenvinphos + Dichlorvos + Alkyl + Xylol; Chlorpyrifos + Cypermethrin hi-cis; Fipronil; Chlorpyrifos; Cypermethrin + Chlorpyrifos + Citronelal; Trichlorphone + Cumaphos + Cyfluthrin; and Diazinon –, determined higher mortality rates, configuring a susceptibility status (WHO),38 therefore, they were efficient in killing R. sanguineus larvae, in the dosages recommended by the laboratories that manufacture these products (Table 1).
The products that proved to be ineffective had their respective lethal concentrations calculated. The ideal concentrations to kill 99% (CL99) of R. sanguineus larvae, referring to products Deltamethrin; Cypermethrin; Permethrin; Cypermethrin + Piperonyl Butoxide; and Amitraz (after 24 and 48 hours of exposure), were respectively 35.2; 21; 7.85; 3; 3.65; and 1.55 times greater than the dosages recommended by the manufacturing laboratories (Table 2). However, it is important to point out that the dosage proposed by the acaricide manufacturers is due not only to the in vitro acaricidal efficiency of the product, but also to the absence of side effects harmful to the health of the animals treated, and that, therefore, for safety reasons, it should not be recommends the application of higher doses of these products, in order to avoid intoxication of these hosts and also of the man (the applicator of the acaricide in the animals), and the extremely harmful residual accumulation of these pesticides in the environment and food chain.
|
Acaricidal formulations |
Concentration recommended (%) |
LC50 (CI 95%) |
LC99 (CI 95%) R2 |
|
|
|
|
|
|
|
|
Deltamethrin |
0,2 |
0.060 (0.05–0.07) |
7.04 (3.64–17.4) |
0.9287 |
|
Cypermethrin |
0,1 |
0.060 (0.05–0.07) |
2.10 (1.22–4,51) |
0.9271 |
|
Permethrin |
0,2 |
0.140 (0.13 –0.17) |
1.57 (1.00–3.11) |
0.8672 |
|
Cypermethrin + Piperonyl butoxide+ citronellal |
0,1 |
0.062 (0.05–0.067) |
0.30 (0.24–0.40) |
0.8285 |
|
Amitraz (24h) |
0,2 |
0.025 (0.02–0.03) |
0.73 (0.52–1.13) |
0.9481 |
|
Amitraz (48h) |
0,2 |
0.06 (0.06–0.07) |
0.31 (0.26–0.40) |
0.9786 |
Table 2 Acaricidal products that demonstrated resistance status by Rhipicephalus sanguineus larvae, with their respective concentrations (%), recommended by the manufacturer, LC50, LC99 and R2, in Goiânia, Goiás State, Brazil
LC50: Lethal concentration 50%; LC99: Lethal concentration 99%; C: Confidence Interval; R2: Coefficient of determination.
To verify the degree of relationship between concentrations and mortality, the coefficient of determination (R2) was calculated, which represents the result of the existing variation between the independent variables (Concentrations) and dependent variables (Mortality), that is, the closer to 0.1 (100%), the greater the homogeneity of the concentrations with the respective mortalities.31 When analyzing the R2 values obtained, it was found that the increase in concentrations of acaricides, Deltamethrin, Cypermethrin, Permethrin, Cypermethrin + Piperonyl butoxide, and Amitraz, relative to 24h of exposure, interferes in 92.87%; 92.71%; 86.72%; 82.85%; 94.81%, respectively, in the mortality of R. sanguineus larvae, and in 48 h of exposure, referring to Amitraz, 97.86% (Table 2 and Figure 1).
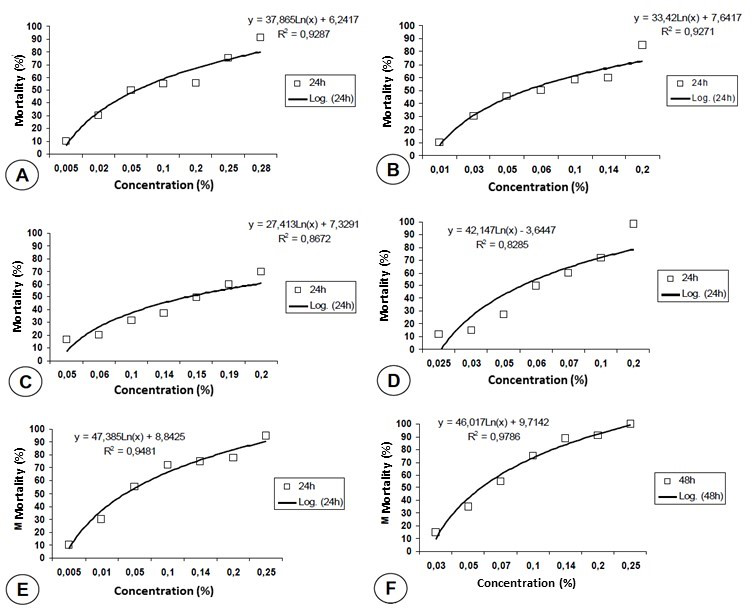
Figure 1 Mortality of Rhipicephalus sanguineus larvae by the action of different concentrations of the following acaricides: (A) Deltamethrin, (B) Cypermethrin, (C) Permethrin, (D) Cypermethrin + Piperonyl Butoxide, (E) Amitraz (24h), and (F) Amitraz (48h), in Goiânia, Goiás State, Brazil. The squares represent the mean mortality percentages obtained with the different concentrations and the trend line is derived from log-probit analysis. R2 = coefficient of determination; y = straight line equation.
Low acaricide efficiency or ineffectiveness was observed for all products composed only of pyrethroids, evaluated in this work, on larvae of R. sanguineus from Goiânia. These data are consistent with those of Fernandes and et al.,6,22,23,25 who had previously reported the loss of larvicidal efficiency of pyrethroid-based products, also in populations of R. sanguineus from this municipality, now confirmed with the results of the present Work. This becomes an obstacle for the choice of less toxic acaricides for dogs and for humans (dog breeders), to control this tick, since pyrethroids are considered, among other toxicological groups, the less toxic to mammals and therefore safer to use.40
The Fipronil-based product, despite having demonstrated acaricidal efficiency, presents an obstacle to its use due to the still very high cost for most dog breeders in the country. The acaricide containing Coumaphos, or the combination of Chlorpenvinphos and Dichlorvos, although efficient, should be avoided due to its high toxicity to mammals, characterized as highly dangerous by the WHO,41 due to residual accumulation in the food chain and the environment.42,43 However, products with formulations composed of associations of acaricide bases, organophosphates and pyrethroids, proved to be a reasonable alternative for chemical control, once resistance to formulations containing only pyrethroids was verified. This is because they determined the mortality of 100% of the larvae in a less toxic way than the formulations consisting exclusively of organophosphates, in addition to having a more accessible cost. These results are consistent with those reported by FAO,44 which showed the different mechanisms of action and metabolism of these two classes of acaricides and the potentiation of pyrethroid action promoted by the presence of organophosphate.
The growing number of records of ineffectiveness of commercial chemical acaricides as a result of the development of tick resistance to various acaricide bases, such as pyrethroids, formamidines, organochlorines and organophosphates, has been a reality in Brazil, Latin America and several other countries.33,34,44,45
Fernandes and et al.,6,22,23,25 had already reported resistance of R. sanguineus to the synthetic pyrethroids Deltamethrin and Cypermethrin, also in Goiânia, Goiás – a picture consistent with the results found in the present study, which also revealed resistance of R. sanguineus larvae to these pyrethroids, and, now, also to Permethrin. These data confirm the increasing loss of effectiveness of pyrethroid acaricides, in the dosages recommended by the manufacturing laboratories for the control of R. sanguineus.
In the Brazilian State of Paraíba,46 as well as in Zaragoza,47 Spain, studies pointed to Amitraz as an acaricide base with excellent activity in the control of the brown tick of dogs, a fact that differs from what was found in the present study, that present larvicidal resistance to the product after 24 hours of exposure, and a low efficiency after 48 hours of exposure, results that are concordant with those presented by Miller et al.45 with strains of R. sanguineus from Panama, also resistant to Permethrin and Amitraz.
These data reveal a rather worrying picture, that of the progressive loss of efficiency of synthetic chemical acaricides, which still constitute the most used control method against ticks. The excessive and thoughtless use of acaricides, in inappropriate dosages or application methods, selects tick populations that are increasingly resistant to synthetic acaricides. There are at least three mechanisms for the development of resistance to acaricides that have been revealed by the researchers: "metabolic resistance"; "resistance by target site modification"; and "resistance due to reduced penetration of the acaricide". In short, what can be seen is that no matter how much new chemical acaricide bases are developed, mutant genes always appear in tick populations, which are hereditarily transmitted to descendants by ticks that survive the interaction with acaricides. These mutated genes are initially uncommon but increase in frequency over time. Thus, the resistant population increases significantly, leading to a loss of efficiency of the acaricides.48,49
More modern acaricide bases, which are still efficient in certain countries in controlling R. sanguineus, may become ineffective in other countries, and vice versa. Fipronil, for example, proved to be effective in the present study to combat larvae of R. sanguineus, but in Florida, USA, it was estimated that larvae of this species have tolerance to this acaricide, and also resistance to Permethrin.27 Resistance developed by this ixodid to ivermectin was evidenced in Mexico,28 and also in India, in addition to the fact that resistance to Deltamethrin and Cypermethrin was also observed in this country.4,29
In view of this, an alternative measure to synthetic chemical acaricides, to be added to a rational integrated system of ticks control measures,50 is the development of anti-tick vaccines. There are vaccines on the market (Bm86) with antigens related to intestinal membrane proteins of the cattle tick, however, they have low lethality at adult stages (20-30%) and are still costly for the producer. According to the FAO,44 these factors hinder its practical viability in Latin American countries. Nevertheless, research points to new paths for the development of vaccines that are more efficient than those available on the market, employing the inhibition of genes responsible for the synthesis of anti-hemostatic agents present in the saliva of ticks, essential for successful feeding and survival of the tick51, or through RNA interference for tick genetic manipulation methods52 Although technological advances tend to reduce production costs, and many promising antigens for vaccines against ticks have been discovered through genomic, transcriptomic and proteomic studies, few of them have been investigated in depth as to their true potential. Thus, in practice, vaccines against several species of ticks are not yet commercially available.53
As another alternative to conventional chemical control of ticks, promising results have been obtained with the use of organonatural substances – “green acaricides” – in the control of vectors of medical and veterinary importance, including R. sanguineus.30,35,36,52-54 Botanical substances with acaricidal properties meet the need for less polluting and biodegradable compounds, which leads to a reduction in the aggression to the environment exerted by conventional chemical acaricides and provide an environmental awareness of the need to preserve natural resources.
This study is part of the stricto sensu postgraduate degree in Tropical Medicine of WBD, at the Laboratory of Medical and Veterinary Arthropodology (LAMV), Federal University of Goiás (UFG), Goiânia, Goiás, Brazil, scientifically supervised by Professor Fernandes FF, Head of LAMV. The authors would like to thank the financial support provided by the CNPq (Brazilian Council for Scientific and Technological Development), and Sectec-GO (State Secretariat for Science and Technology of Goiás), employed in the acquisition equipment and research materials for carrying out this study; as well as to Research Support Foundation (Funape) and Coordination for the Improvement of Higher Education Personnel (Capes) for the scholarships awarded, respectively, to WBD and RNL.
The authors declare that there is no conflict of interest.

©2022 D´Alessandro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.