Journal of
eISSN: 2373-437X


Mini Review Volume 3 Issue 4
Department of Microbiology, Assam Don Bosco University, India
Correspondence: Prosun Tribedi, Department of Microbiology, Assam Don Bosco University, Airport Road, Azara, Guwahati-781017, Assam, India
Received: July 10, 2016 | Published: September 30, 2016
Citation: Tribedi P (2016) Microbial Socialism: a Key Way to Stabilize Complex Biofilm Structure. J Microbiol Exp 3(4): 00098. DOI: 10.15406/jmen.2016.03.00098
Biofilm is a sessile microbial community characterized by cells that are attached to an abiotic or biotic surface and embedded in a matrix of extracellular polymeric substances (EPS) produced by themselves. Microorganisms often develop multilayer biofilm for getting better protection and stability under different environmental stresses. However, biofilm also experiences severe problem in nutrient exchange, diffusion of oxygen and removal of toxic byproducts because of its multilayered structure. In order to gain stability in biofilm, microorganisms in biofilm introduce considerable changes including in its phenotypic and genotypic pattern, making the microbial biofilm population more heterogeneous in terms of functional richness, phenotypic plasticity and metabolic diversity leading to the formation of a social, functionally diverse interconnected microbial community inside the biofilm. Formation and persistence of a biofilm is a complex and dynamic process that needs to be studied in detail to open a new axis of customizing measures against biofilm World.
Keywords: biofilm, microbial communication, stabilization of microbial community
Microorganisms in environment exist in two forms, one in planktonic form and another in biofilm form. Planktonic form represents the free living discrete microorganisms whereas biofilm form reveals complex microbial clusters adhered on a surface.1 Biofilms are groups of microorganisms residing within a self-produced matrix of extracellular polymeric substances which get colonized to several living and non-living surfaces.2,3 Microbial biofilms are ubiquitous in every environment including soil and water bodies. Both homogenous and heterogeneous types of microorganisms can exhibit biofilm formation.4 Extracellular polymeric substances (EPS) have been synthesized by the microorganisms when they form biofilm in order to hold the complex microbial network efficiently.5 It has been observed that several biomolecules including sugars, proteins and lipids are present in EPS6 whereas the polysaccharides are found to be the most abundant biomolecules (40-95%) compared to others.7 Yang et al.,8 also found that exogenous-DNA (e-DNA) was one of the major matrix components in P. aeruginosa biofilms, functioning as an intercellular connector. They also supported the concept of the stabilizing role of e-DNA for the biofilm matrix. Production of EPS by bacteria in culture or in aggregates depends on several factors such as type of microbial species, phases of growth, nutritional status and the environmental conditions.9 Compositional analysis and extraction methods of EPS from pure bacterial cultures, biofilms, activated sludges and bio-granule have been reported by Pal and Paul9 wherein they showed that the composition of EPS varies considerably depending on the given condition. EPS holds the structural integrity of biofilms, and are considered as the fundamental component that determines the physiochemical properties of a biofilm. Biofilms are of two types: single layered biofilm and multilayered biofilm, which depends on the interaction between the surface where the microorganism adhere and constituent cells which adhere.10 Microbial biofilm develops through a series of consecutive stages such as initial reversible attachment, irreversible attachment, maturation and dispersion.11,12 The stages of microbial biofilm formation have been presented in Figure 1. In the first stage, planktonic microbial cells attach to the surface either by physical forces or by microbial appendages such as Pilli or flagella.13 Then the microbial attachment to the concerned surface becomes irreversible from initial reversible binding. The next phase in biofilm formation is the maturation. In this phase, microbial cells start communicating among each other by the production of auto-inducer molecules14,15 that results in the expression of biofilm specific genes. In this stage, microorganism secretes extracellular polymeric substance (EPS) to stabilize the biofilm network. The next stage is the dispersion which marks detaching of the biofilm and return of sessile cells to the motile form.16 In this stage, biofilm spreads and colonizes to the new surfaces. Existing literature showed that different factors such as the hydrodynamics of the bulk fluid,17,18 the nature of the substratum,19 species composition in biofilm, nutrient variation5 and nutrient availability18 influence biofilm formation, but it is still not clear exactly how these factors interplay, or which factors dominate most in modulating biofilm formation. It has been noted that the surface characteristics of bacteria lead to repulsion.10 For instance, the chemical properties of the cell wall of gram-negative bacteria are generally determined by the O antigen, which is generally having negative charge in nature. For the formation of multilayer biofilm, this repulsive force due to similar charge among microorganisms has to be neutralized. This negative charge used to be masked by the addition of divalent cations.20 These metal ions not only neutralize the repulsive forces but also can activate certain enzymes in EPS by acting as co factors. There are many sensing systems including two-component systems (TCS), extra cytoplasmic function (ECF) and quorum sensing (QS) events that can regulate the development of microbial biofilm formation.4 QS is a process of bacterial communication through the use of auto-inducers or pheromones. When the microbial population reaches a threshold density, then the microorganisms secrete signalling molecules known as auto inducers that can act on the receptor of microorganisms and activates the expression of genes required for biofilm formation.21 For gram positive bacteria, oligopepetide has been considered as the auto inducer whereas N-Acyl homoserine lactone (AHL) has been considered as auto inducer for gram negative bacteria.4 Microbial biofilm has been strongly associated with microbial pathogenesis, wastewater purification, bioremediation of heavy metals and recalcitrant molecules etc. The aim of this manuscript is to discuss about how the microorganisms behave socially to stabilize the complex microbial biofilm network.
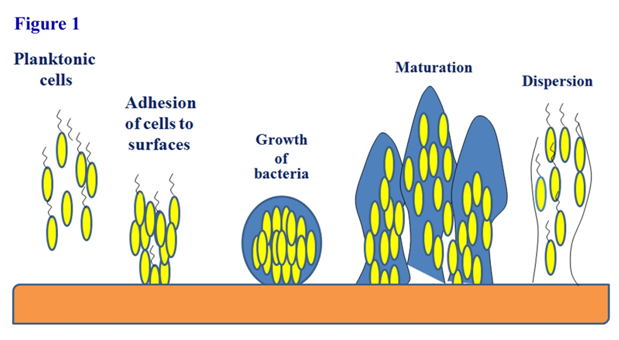
Figure 1 Formation of a biofilm. Planktonic cells attach first reversibly to the surface and then become associated irreversibly which leads to the formation of a colony on the surface. Quorum sensing and other signalling events lead to maturation and stabilization of biofilms. Thereafter, microbes inside the biofilm disperse for colonization to a new surface.
Biofilm represents a dense microbial cluster of microorganisms which assemble together in a very complex process. In most of the cases, biofilm develops multilayered association. Thus, in case of multilayered biofilm, the microorganisms present on top of the biofilm gets oxygen freely but the microorganisms in bottom layer hardly get oxygen. Multilayered network of biofilm often creates stress for the microorganisms in it by accumulating toxic by-products and preventing uniform flow of nutrients to each cell in biofilm. The water present in the matrix is efficiently trapped by hydrogen bonding with the hydrophilic moieties in EPS.22 Thus, in this aqueous environment, the water channel provides effective means of transporting nutrient to the cell as well as removal of toxic metabolites by the cells in biofilm.23 Analysis of the EPS coating present in the biofilm has lead to the discovery that biofilms are technically hydrogels which exhibit visco-elastic behavior24 in order to help the biofilms to withstand mechanical stress.
Biofilm represents a composite association of microorganisms in a given ecosystem, and its formation is a microbial survival response to antagonistic environment.25 Microorganisms are known to be capable of changing their structural, genetic and physiological activities through biofilm formation as biofilm allows survival under varied ecological conditions.26 Biofilm communities always showed different gene expression pattern compared to their planktonic form.4 Such alteration in activities for better survival in a given habitat is known as adaptability. Adaptation of bacteria to new environment often results in the acquisition of genetic trait via horizontal gene transfer rather than accumulation in modification of gene function by mutation.27 The mobile genetic element which mediates the horizontal gene transfer includes conjugative plasmid, transposons or bacteriophage.27 This horizontal gene transfer is important for generating genetic diversity in biofilm that can make biofilm functionally diverse and independent.
When bacteria are growing within a biofilm, gene expression pattern changes that result in genotypic and phenotypic heterogeneity within the microorganisms in biofilm.15,28 This has been considered as specialization or division of labour similar to cellular differentiation seen in multicellular organisms. Biofilm bacteria can sense their background, and this enables them to adjust their metabolic processes to maximize the use of available substrates26 and to protect themselves from detrimental stress conditions. Bacterial cells do not differentiate, rather they respond to their environmental surroundings by adapting their gene expression to meet their own needs for survival. If however, bacterial cell got detached from the biofilm and face a new environment, then the detached microbial population quickly adapt to that new environmental surroundings and exhibit considerable phenotypic changes.28
It has also been observed that bacteria exhibit altruistic behaviour in biofilms. In this altruistic behaviour, the actor microorganisms lose its own fitness and increase the fitness of recipient microorganisms29 and this is considered as one of the features of sincere cooperation in microbial community. The biofilm microbial population get benefitted from a number of properties of a communal existence including favourable division of the metabolic burden, gene transfer, and selfless behaviour.28 A popular concept of this division of labour is accomplished through intercellular communication. In this context, bacteria secrete substances referred to as auto inducing signals which allow the bacteria to communicate with one another.4 Ecologists have also recognized that the stability of many types of biological communities including microbial community is enhanced by diversity and heterogeneity.30 For example, communities having mono species are more susceptible to environmental perturbations than communities having diverse species. This phenomenon has been nicely explained by the ‘‘insurance hypothesis’’ in biological science. Insurance hypothesis represents that the presence of diverse subpopulations of microbial species in an ecosystem increases the range of conditions in which the community as well as the ecosystem can survive.30 Insurance effects could be of great benefit to biofilms because, like other communities, their long-term accomplishment depends on their ability to withstand efficiently against changing environmental conditions. Thus, it is more precise to say that the microorganisms become more social when they are in biofilms. This communal character makes biofilms as interactive social microbial community.
Microorganisms often develop biofilm to get protection from external assaults. Microorganisms in biofilm display socialism efficiently in order to stabilize the biofilm and make it functionally versatile. More metabolically diverse, functionally rich, genotypically and phenotypically diverse microbial biofilms develop a cooperative, socially relevant microbial biofilms which can sustain for long period of time over any surface under a given environmental condition. Therefore, detail understanding is required for the series of events that trigger the development of communal, interconnected microbial communities inside biofilms.
None.
Authors declare there are no conflicts of interest.

©2016 Tribedi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.