Journal of
eISSN: 2373-437X


Research Article Volume 8 Issue 2
Department of Microbiology, Jahrom Branch, Islamic Azad University, Iran
Correspondence: Houshang Jamali, Department of Microbiology, Jahrom Branch, Islamic Azad University, Jahrom, Iran, Tel 004915759051097
Received: April 04, 2020 | Published: April 21, 2020
Citation: Jamali H, Iran D. Investigation on urinary tract infections following C-section and assessment of CTX-M and TEM genes prevalence in Escherichia coli and Klebsiella causing the infections in Lamerd. J Microbiol Exp. 2020;8(2):73-78. DOI: 10.15406/jmen.2020.08.00288
Background and objectives: Antibiotics are usually prescribed by surgeons before and after surgery to prevent postoperative infections. Prophylaxes used for cesarean section in Lamerd are cefazolin and cephalexin. According to studies carried out, urinary tract infections can also occur in spite of the above prescribed antibiotics. So, this research was carried out to determine those strains resistant to the mentioned antibiotics and also to identify genes causing resistance in antibiotic-resistant strains.
Methods: 140 cases of mothers who had cesarean section (C-section) were considered in this study. The research began in April and lasted till July 2014 for case selection and sampling. It was emphasized to those women delivered via C-section to refer to laboratory 15 days after delivery. UA-UC tests were done on them. Phenotypic confirmatory test was performed for cefazolin and cephalexin antibiotic-resistant strains in the presence of ceftazidime, ceftazidime/clavulanic acid and cefotaxime, cefotaxime/clavulanic acid discs. The presence of CTX-M and TEM genes in the resistant strains were examined finally by performing PCR test.
Results: Of 140 studied C-sections, 10 cases of urinary tract infections including 3 cases of E. coli (2.1%), 1 Klebsiella (0.7), 2 cases of Proteus (1.4%) and 4 cases of Staphylococcus (2.8%) were observed. According to phenotypic confirmatory test, Escherichia coli and Klebsiella strains were isolated as strains containing ESBL for next studies. Finally, according to PCR test for Escherichia coli and Klebsiella isolates, all of them contained CTX-M and TEM genes.
Conclusion: It is necessary that microbial culture and antibiogram tests to be done before administering any antibiotics, in order to prevent the spread of strains containing ESBL with proper prescription of antibiotics.
Keywords: urinary tract infections, C-section, CTX-M, ESBL, TEM
C-section is a major surgery and several factors including, age, physiological changes of organs during pregnancy, excessive bleeding after C-section, use of catheter, duration of hospitalization and immune system disorders in mothers after C-section can be grounds for postoperative urinary tract infections.1,2
Urinary tract infections are the most common bacterial infections during pregnancy. Although asymptomatic bacteriuria is the common case, symptomatic infection may cause cystitis and/or may cause pyelonephritis by engaging collecting system, pelvis and renal parenchyma. Organisms that cause urinary tract infections are the ones that form the normal flora of perineum.2
In order to prevent infections after C-section, single-dose antimicrobial prophylaxis is done in almost all cases. The American College of Obstetricians and Gynecologists (ACOG, 2003) recommends such prophylaxis for women who are at high risk of postpartum infections. This single-dose antimicrobial prophylaxis, more than any other action in the last 30 years has reduced the incidence and severity of infections after C-section.2
Escherichia coli genus at least contains 5 species that E. coli is more common among them.3 The bacteria are gram-negative and non-spore forming. Some of them have capsules or microcapsules or slime layer. Pili, which have the ability of co-agglutination are found in most of them and they are mannose sensitive (type 1) and mannose resistant types (type 2). Type 1 fimbriae exist in most of E. coli samples. Fimbriae involve in bacterial pathogenesis and attachment of bacteria to urinary tract epithelial and mucosal surfaces is caused by them. More than 85% of urinary tract infections are caused by this organism and it is considered as the first cause of urinary tract infections in pregnant women.4
Three genera including, Klebsiella, Enterobacter and Serratia are known as Klebsiella genus. Like entire family, they grow on blood agar and EMB agar and MacConkey’s agar culture mediums. Most members of this genus have red and/or lactose-positive colonies.5 The most important bacterium of this group is Klebsiella pneumoniae which causes pneumonia. Klebsiella pneumoniae may be involved in incidence of infected wounds and urinary tract infections.3 Klebsiella pneumoniae is the cause of bacteremic urinary tract infections with focal lesions and it is also important in nosocomial infections due to drug resistance. Klebsiella pneumoniae produces pink colonies (lactose positive) and phlegmatic or mucoid colonies (due to the production of thick capsule) on MacConkey’s agar culture medium.3
A new issue raised about bacteria is the emergence of extended-spectrum β-lactamase (ESBL) enzymes. ESBL-containing bacteria are resistant to many antibiotics. By employing different methods, bacteria can protect themselves from harmful effects of antibiotics. Production of β-lactamase enzymes is one the most important mechanisms used against β-lactam antibiotics in gram-negative bacteria.6 In fact, incidence of point mutations in beta amino acid sequence causes derivation and creation of TEM1 and TEM2 primary lactamases.7-9 Based on primary structure, β- lactamases are divided into 4 molecular classes of A to D. Class A, C and D β-lactamases are serine-based enzymes and class B β-lactamases is a metalloenzyme containing zinc. Classification of β-lactamases began when cephalosporins were distinct from penicillinases and divided into four functional groups. First group: cephalosporinases that not inhibited well by clavulanic acid and are belonged to molecular class C. Second group: contains penicillinase and cephalosporinase or both that are generally inhibited by β-lactamases inhibitors. The group is belonged to molecular class A or D. SHV, TEM and CTX-M β-lactamases of the second group are always on the rise and are divided into several groups based on their substrates.
β-lactamases are divided into four groups in latest studies: A) ESBLs, B) inhibitor-resistant class A derivatives of β-lactamases, C) plasmid-mediated AmpC β-lactamases and D) carbapenem-hydrolyzing-β-lactamases. ESBLs are a class of β-lactamases that have special importance in antimicrobial therapy. The enzymes are capable of complete hydrolyzing of Oximino β-lactams, which exist in the building of third-generation cephalosporins. These enzymes, which were most from TEM and SHV types, were first detected in 1980s. As a result, point mutations in main enzymes have been developed without broad-spectrum activity. CTX-M family was first reported from ESBLs in Germany in 1989 and then spread around the world.10-12
CTX-M β-lactamases are increasingly common in E. coli and Klebsiella pneumonia species13 and are associated with prevalence of urinary tract infections. SHV, TEM and PER enzymes have been detected by phenotypic methods in studies performed in Iran.14-16 Molecular methods can also identify genes producing the enzymes.16 Studies determined that 72 samples (77.4%) of 93 samples were CTX-M positive by PCR assay.17 It was found in a study conducted by Burcu Bali that among 44 E. coli samples 72.72% (32 samples) and 22.72% (10 samples) had TEM and CTX-M genes, respectively.18 Since the use of prophylactic antibiotics are seen in C-sections, the following research is conducted for comparison study of single-dose and multiple-dose antibiotics administrations in prevention from prevalence of infections after C-section.
140 cases of women who had undergone C-section from April to July 2014 were studied in this research. The study was aimed at investigating the prevalence of antibiotic resistance genes. So, a UA-UC test was performed on the studied samples before surgery.
Prophylaxis prescribes for all surgeries, especially in C-sections. Since the aim of this study is investigation on infections following C-sections despite antibiotic prophylaxis, a UA-UC test for the cases with C-section delivery was done again 15 days after the patient discharge from hospital. This was performed by coordination with relevant personnel and physicians. The reason for considering a period of 15 days was that the patients had received cephalexin 500 mg for 10 days after discharge and they had also received 2 g of cefazolin IV during the hospitalization period. At the time of discharging from the hospital, it was emphasized to the patients to be present in laboratory for morning urine sampling at the next 15th day of hospital discharge and to avoid use of antibiotics without coordination with their gynecologists.
The urine samples were cultured on blood agar and EMB medium cultures. Differential biochemical tests were used to identify types of bacteria following bacterial growth on the above-mentioned cultures. Antibiogram test was done for bacteria in the next stage and the desired bacteria were kept in Skim milk broth culture at -70˚C.
The antibiogram test was performed as following: a standard bacterial suspension was firstly prepared in accordance with a 0.5 McFarland concentration. After the spread of bacterial suspension on Mueller-Hinton agar medium using a sterile swab, antibiotic discs containing, nalidixic acid, cefazolin, cefixime, ciprofloxacin, nitrofurantoin, gentamicin, cotrimoxazole, ceftriaxone and cephalexin were placed in continuous on Mueller-Hinton agar medium at distance of 2.5cm from each other.
The cultures were incubated for 24 hours at 37˚C. Then, the diameter of the halo around the antibiotic discs where no bacterial growth had occurred was measured with a ruler. According to manufacturer’s instruction of the discs, the desired samples were reported as resistant (R), semi-sensitive (I) and sensitive (S). After being done the above-mentioned test, the presence of ESBL genes in all strains that their tests were positive should also be approved and so PCR test was necessary for that purpose. PCR testing procedures are as follow:
A culture on Mueller Hinton agar medium was prepared at first from the stored samples in Skim milk broth medium. The samples were incubated for 24 hours at 37˚C. Boiling method was used for DNA isolation and purification. In this method, the bacterial colonies were dissolved in 500 microliter of distilled water inside a micro-tube. After this stage, the sample was boiled for 15min at 100˚C and centrifuged for 14000 rpm for 10min. Finally, PCR test was performed to identify CTX-M (550 bp) and TEM (847 bp) genes. PCR reaction was done in a final volume of 25 microliter, according to the following conditions:
50 mMTris. Hcl, 50 mMKcl, 0.2 mM dNTP, 10 picomole of each primer, 200 ng of DNA template accordance with time and temperature conditions presented in Thermoseeker device. Time and temperature conditions of Thermoseeker and the nucleotide sequence of primers are listed in Table 1 and Table 2.
|
Temperature |
Time |
|||
˚C |
min |
||||
Rows |
Stages |
CTX-M |
TEM |
CTX-M |
TEM |
1 |
Initial denaturation |
94 |
94 |
4 |
5 |
2 |
Denaturation |
94 |
94 |
1 |
1 |
3 |
Annealing |
60 |
61 |
0.5 |
1 |
4 |
Extension |
72 |
72 |
1 |
1 |
5 |
Final extension |
72 |
72 |
5 |
5 |
Table 1 The conditions used for PCR analysis of ESBL gene
Gene names |
Nucleotide sequence of used primers from 5→3 |
Weight of product (bp) |
Reference |
CTX-M – F |
TTTGCGATGCAT ACC AGT AA |
550 |
43 |
CTX-M–R |
CGATATCGTTGG TGGTGCCAT |
||
TEM –F |
ATGAGTATTCAACATTTCCG |
847 |
37 |
TEM-R |
CTGACAGTTACCAATGCTTA |
||
Table 2 The used sequence of primers for PCR analysis
To identify the presence of CTX-M and TEM genes, the PCR products were electrophoresed on 2% agarose gel. 6 microliter of the PCR products with 4 microliter of loading buffer were mixed with each other and poured in the well. The electrophoresis device ran at 100 V and 50 mA and electrophoresis was performed. The gel was placed in ethidium nromide for 15 min and then the presence of bands was evaluated using gel documentation device. Finally, all the obtained data in this study were analyzed using SPSS statistics 20.
Among 140 mothers who had referred to the laboratory 15 days after C-section delivery, 10 patients (7.1%) were suffered from UTI despite Prophylaxis with cefazolin and cephalexin after surgery. Among the identified patients (10 mothers), 3 cases (2.2%), 1 case (0.7%), 2 cases (1.4%) and 4 cases (2.8%) were recognized to be infected by E. coli, Klebsiella, Proteus and Staph, respectively. After that the patients with UTI originated from E. coli and Klebsiella were selected and the percentage of E. coli and Klebsiella resistance to various antibiotics were studied and the results are expressed in Table 3.
Antibiotics |
E. coli |
Klebsiella |
|||
Name |
Abbreviations |
R |
S |
R |
S |
Cefixime |
CFM |
10 |
0 |
10 |
0 |
Cefazolin |
CZ |
100 |
0 |
100 |
0 |
Gentamicin |
GM |
33.3 |
66.7 |
0 |
100 |
Nitrofurantoin |
FM |
0 |
100 |
0 |
100 |
Ciprofloxacin |
CP |
33.3 |
66.7 |
100 |
0 |
Cephalexin |
CTX |
100 |
0 |
100 |
0 |
Cotrimoxazole |
SXT |
100 |
0 |
100 |
0 |
Nalidixic acid |
NA |
66.7 |
33.3 |
100 |
0 |
Cephalexin |
CN |
100 |
0 |
100 |
0 |
Table 3 The percentage of resistance in E. coli and Klebsiella isolated by the type of bacteria to the prescribed antibiotics
Thus, according to the above results, an issue that medical community should be concerned about is prescription of proper antibiotics for patients. In cases that prescription of antibiotics and prophylaxes is not systematically and scientifically done before and during surgery, society will be gradually the testifier of a wide range of microorganisms resistant to antibiotics.
Following the confirmed test for the presence of ESBL genes, PCR test was performed to identify TEM and CTX-M genes on E. coli and Klebsiella isolated from the patients and the following results were obtained (Figures 1-3).
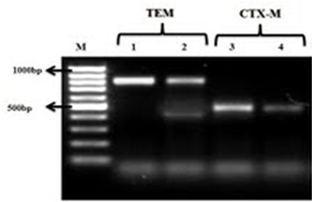
Figure 1 The PCR results for E. coli (1); 1 and 3 markers are positive controls for TEM and CTX-M, respectively; 2 and 4 markers are TEM and CTX-M genes, respectively.
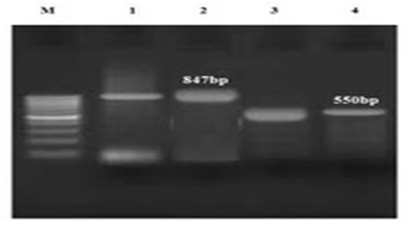
Figure 2 The PCR results for E. coli (2); 1 and 3 markers are positive controls for TEM and CTX-M, respectively; 2 and 4 markers are TEM and CTX-M genes, respectively.
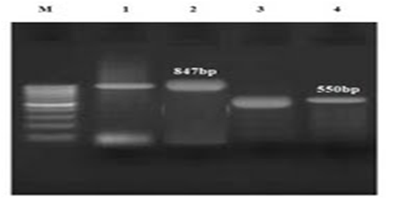
Figure 3 The PCR results for E. coli (3); 1 and 3 markers are positive controls for TEM and CTX-M, respectively; 2 and 4 markers are TEM and CTX-M genes, respectively.
According to the above results, in case that the effect of antibiotics on isolated strains was studied, the following results obtained:
Klebsiella was quite sensitive to gentamicin and nitrofurantoin and E. coli was absolutely sensitive to nitrofurantoin. This means that the mentioned strains did not show resistance to these antibiotics. However, Klebsiella was quite resistant to cefixime, cefazolin, ceftriaxone, ciprofloxacin, cortimoxazole and nalidixic acid. But despite that E. coli was absolutely sensitive to nitrofurantoin, it was absolutely resistant to cefixime, cefazolin, ceftriaxone and cephalexin and showed little resistance to gentamicin and ciprofloxacin. It had little sensitivity (approximately 33%) to nalidixic acid and due to increasing prevalence of resistance to this antibiotic, its prescription is not recommended.
Resistance to antibiotics has gradually spread. Concluded from extensive studies of researchers in this field, many of micro-organisms, especially those responsible for nosocomial infections have increasingly shown resistant to a variety of antibiotics. So, this issue becomes clear according to the study conducted in the city of Lamerd and according to the results of previous studies (Figure 4).
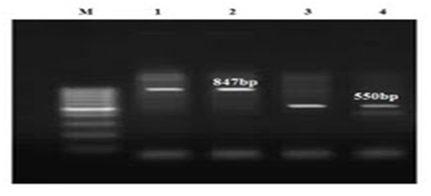
Figure 4 The PCR results for Klebsiella; 1 and 3 markers are positive controls for TEM and CTX-M, respectively; 2 and 4 markers are TEM and CTX-M genes, respectively.
As it became clear, all isolated E. coli and Klebsiella absolutely contain CTX-M and TEM genes (Figure 5) and this proves that the isolated strains are absolutely resistant to cephalosporins. In other words, no impressive work had been done on the patients who received cefazolin and cephalexin. This result is also considered, given the prevalence of UTI (7.1%) in the studied group, and the presence of other bacteria such as Staphylococcus aureus and Proteus in creation of infections.
Infection is the most common and important complications after C-section. Prescription of antibiotics has been considered in preventing this problem. The results of this study showed that the development of endometritis in a single dose group was 1.3% and none of the patients in multi-dose group was diagnosed with this condition. The prevalence of surgical site infection in the single-dose and multi-dose groups was 8% and 9.3%, respectively. The rate of postpartum febrile morbidity in the single-dose and multi-dose groups was 4% and 5%, respectively. No significant difference was observed statistically between the two groups in terms of the above-mentioned complications. Considering the results of this study, it seems that in the majority of patients who are delivered by C-section, prescription of cefazolin in a single-dose and multiple-dose has no difference in preventing endometritis, surgical wound infection and febrile complications.
Impact of intravenous cefazolin on low-risk postpartum infectious complications was investigated in another study on 257 patients hospitalized in Mostafa Khomeini Hospital and HazratZeinab Hospital (SA) in Tehran. The rate of urinary tract infections in the group received cefazolin was 1.6% and infection rate in patients who did not receive the medication was 3.1%.
Note that asymptomatic UTI in pregnant women can also be the source of infections after C-section. In a study conducted by19 in Hamedan, 377 pregnant women were checked in terms of the prevalence of asymptomatic urinary tract infections. In the women who infected by E. coli and Klebsiella pneumonia resistant to cefixime and cefazolin, the prevalence in patients with E. coli infection and resistant to cefixime and in patients resistant to cefazolin was reported 42.6% and 53.8%, respectively; the prevalence in patients with Klebsiella pneumonia infection and resistant to cefixime and in patients resistant to cefazolin was reported 0% and 33.3%, respectively. As can be seen, some resistant patients to cefixime and cefazolin are observed among the isolated patients and in this case if physician prescribes each of these two antibiotics to prevent postpartum infections without performing antibiogram test, no treatment process is actually done on them.
One of the topics discussed today in the field of drug resistance is emergence of ESBL strains, which have drug-resistant genes, including CTX-M, SHV and TEM genes. Most of the resistances are caused by the movement of plasmids, transposons and chromosomal changes of micro-organisms.
In the present study, all the isolated strains were ESBL type, and this result is consistence with the results derived from investigations of Shahcheraghi et al, in 2010 based on the presence of ESBL strains in Fars Province. In comparison between this study and other studies performed all around the world about CTX-M recipient strains, it can be seen that the prevalence of CTX-M recipient strains is increased by increase of years from 2005. According to the statistics provided in the case of ESBL genes, the prevalence of antibiotic-resistant strains containing CTX-M β-lactamase genes in the United States had increased from 26.6% in 2007 to 43.8% in 2010.20 The prevalence of antibiotic-resistant strains in Korea hospitals was 84.3% in 200521 and in Brazil hospitals was 61.1% in 2008.22
Prescription of antibiotics in normal delivery is much less than cesarean section delivery. So, it seems that prescription of antibiotics during cesarean section is itself a factor for creation of ESBL strains. Due to drug resistance, the strains have unpleasant side effects for the patients who themselves bear the possibility of breeding and feeding of a newborn. Using PCR analysis, the isolated strains in this scheme which were absolutely resistant to cephalosporins contained CTX-M and TEM genes. High prevalence of CTX-M and TEM genes in E. coli and Klebsiella that explained completely in the present study increases the importance of prevention from postoperative infections. It is recommended to physicians to prescribe effective selected antibiotics by implementation of necessary tests and antibiogram test and as previously stated about resistant infections to treatment, to identify those genes causing antibiotic resistance with molecular analysis and PCR tests as well as not to prescribe ineffective antibiotics and to find an effective way to deal with antibiotic-resistant bacteria.
None.
Authors declare that there is no conflict of interest.

©2020 Jamali, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.