Journal of
eISSN: 2373-437X


Research Article Volume 6 Issue 1
1Department of Morphology, Physiology and Basic Pathology, University of Sao Paulo, Brazil
2Department of Stomatology, Public Oral Health and Forensic Dentistry, University of Sao Paulo, Brazil
Correspondence: Marilena Chinali Komesu, Department of Morphology, Physiology and Basic Pathology, Ribeirao Preto School of Dentistry, University of Sao Paulo, Via do Cafe s/n, 14040-900 Ribeirao Preto, SP, Brazil, Tel 55 16 33 15 40 12
Received: August 22, 2017 | Published: February 26, 2018
Citation: Ribeiro AERA, Lourenço AG, Motta ACF, et al. Influence of periodontal condition on levels of human beta defensins 1 and 2 in saliva. J Microbiol Exp. 2018;6(1): 00186. DOI: 10.15406/jmen.2018.06.00186
Many research studies have looked into periodontal disease biomarkers. The aim of this study was to assess salivary levels of human beta defensin 1 and 2 (hBD-1) and (hBD-2) in different periodontal condition. The Periodontal Screening & Recording (PSR) system, a modification of de Community Periodontal Index Treatment Needs (CPITN), was used to evaluate the periodontal treatment needs of 120 patients without any systemic disease. Salivary levels of hBD-1 and hBD-2 were determined by ELISA. Higher hBD-1 levels were detected in the saliva of patients with a PSR/CPITN index of 3 and 4 (7,363±2,837pg/mL) and in those with a PSR/CPITN index of 1 and 2 (7,750±3,477pg/mL) when compared to patients without periodontal disease, with a PSR/CPITN index of 0 (5,644±2,747pg/mL; P=0.013). As with hBD-1, hBD2 levels were also higher in the saliva of patients with a PSR/CPITN index of 3 and 4 (310±98pg/mL) and a PSR/CPITN index of 1 and 2 (318±85pg/mL) when compared to patients with a PSR/CPITN index of 0 (251±87pg/mL; P=0.011). In conclusion, compared to patients without periodontal disease, PSR/CPITN index 0, we verified higher salivary concentrations of hBD-1 and hBD-2 in patients with some degree of periodontal detected by PSR/CPITN between 1 and 4.
Keywords: biomarkers, saliva, periodontal screening and recording, periodontal disease, human beta
CPITN, communiy periodontal index treatment needs; PSR, periodontal screening & recording; hBD 1, human beta defensin 1; hBD2, human beta defensin 2; LPS, lipopolysaccharides; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1 beta; ELISA, enzyme-linked immunosorbent assay; PBS, phosphate buffered saline; ABTS, 2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid
Periodontal diseases are the most frequent oral inflammatory reactions, affecting around 90% of the population worldwide,1,2 leading to local and systemic morbidity. There is great interest in finding biomarkers of periodontal disease as they could shed further light upon its pathophysiology and also upon its relationship with systemic factors.3 Salivary biomarkers may give some important information about unusual cellular activity in periodontal disease, about the effectiveness of periodontal treatment, and also about individual susceptibility to the development of periodontitis.4,5 In addition, to monitor disease progression is a highly skilled action, involving various measures like bleeding on probing, probing depth and attachment loss coupled with radiographic observations. Therefore, it would be desirable to develop biomarkers for early detection of periodontal disease and to identify progression because current diagnostic approaches do not reflect current disease activity but simply assess the cumulative effects of historical tissue destruction.6 Some of these potential biomarkers include human defensins, which are small cysteine-rich cationic peptides, with antimicrobial and immunomodulatory properties, and are found in different tissues of the oral cavity, including salivary glands and gingival tissues.7,8
Recent studies have shown that defensins contribute to antimicrobial defenses, suggesting its capacity to inhibit multiple pathogens in different stages of infection.9 Moreover, these peptides seem to play an important role in adaptive immune system, providing immune surveillance by influencing the maturation of dendritic cells, recruiting immature dendritic and T cells, interacting with chemokine receptor type 6, and activating these cells by toll-like receptor 4.10 Human defensins are classified into alpha, beta, and theta based on their size and cysteine distribution, and six beta defensins have been identified to date,5 but only types 1, 2, and 3 are expressed in tissues of the oral cavity.11 Human beta defensin 1 (hBD-1) is continuously expressed by epithelial tissues12 while human beta defensin 2 (hBD-2) and human beta defensin 3 (hBD-3) are induced by lipopolysaccharides (LPS), peptidoglycans, and inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β).13
Previous studies suggested hBD-1 and hBD-2 expression in gingival tissue, gingival cervicular fluid, and saliva can be altered in several oral inflammatory conditions, including periodontal disease.14 In addition to the continuous search for biomarkers of periodontal disease, simple periodontal screening techniques have been developed for its early detection. The American Dental Association and the American Academy of Periodontology15 developed a simple periodontal screening method known as Periodontal Screening and Recording (PSR) index, which has been widely used and accepted for the early detection of periodontal disease. The PSR index is a modification of the Community Periodontal Index of Treatment Needs (CPITN),16 developed by the World Health Organization to estimate periodontal treatment needs in different population groups.17
The present study assumes that salivary levels of hBD-1 and hBD-2 are influenced by patient's periodontal condition and can be helpful for early periodontal disease detection, and, therefore, aims to assess the hBD-1 and hBD-2 concentrations in different periodontal conditions detected by the PSR/CPITN index, that is used worldwide for the early detection of periodontal disease.
Ethics statement
The study protocol was approved by the Ethics Committee of the School of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil (CAAE protocol no. 0003.0.138.000-10). All patients signed a written informed consent form for their participation.
Patients
A total of 120 patients referred to the Periodontology Outpatient Clinic affiliated with the School of Dentistry of Ribeirão Preto, University of São Paulo, were selected. The inclusion criteria were the following: age over 18 years; no history of systemic diseases, such as diabetes, viral hepatitis, HIV infection; systemic bacterial or fungal infections; dentition containing at least 19 teeth; no previous periodontal treatment in the past 6 months; no antibiotic treatment in the past 3 months; and no history of xerostomia and hyposalivation. The patients were evaluated by anamnesis and clinical examination, during which they were asked about their medical and dental history and had a complete physical extraoral and intraoral examination in order to find any oral mucosal abnormalities.
An experienced examiner (AERAR) estimated the PSR/CPITN index, following the guidelines established by the American Dental Association and the American Academy of Periodontology15 using a periodontal probe, as recommended by the World Health Organization (WHO-621). A specialized periodontal probe with a ball shaped tip having a 0.5 mm diameter was used to examine and score six sites per tooth in each patient sextant on a 0 to 4 hierarchical grading scale, identical to CPITN criteria,16 with only the highest PSR score per sextant recorded for documentation.17 After data collection, patients received oral hygiene instructions, information on their periodontal status and periodontal treatment according to their PSR/CPITN codes. Patients were divided into three different groups based on their periodontal status:
Group 1: Periodontally healthy patients whose six sextants were classified as code 0;
Group 2: Patients with at least one sextant scored as code 1 or 2;
Group 3: Patients with at least one sextant scored as code 3 or 4.
Saliva collection
All patients were instructed not to eat, drink, smoke or brush their teeth at least 1 hour before saliva collection, thereby avoiding contamination risks. Patients were instructed to sit with their heads slightly tilted down and had their saliva sampled from a collector placed below their lower lips. The total amount of saliva collected within a 5-minute period was approximately 5mL. The samples were immediately centrifuged (45,000 rpm for 30 min at 4ºC) and stored at -80ºC until analysis.
ELISA
Salivary levels of hBD-1 and hBD-2 were assessed by enzyme-linked immunosorbent assay (ELISA). Human beta defensin 1 peptide (sc65501 - Santa Cruz, Dallas, USA) was used for hBD-1 analysis and the initial serial dilutions were prepared at a concentration of 800pg/mL. Briefly, 100μL of each concentration was added, in duplicate, per well. The saliva samples were diluted 10 times in phosphate buffered saline (PBS) and transferred in duplicate to ELISA plates (100μL per well). Different hBD-1 concentrations and saliva samples were placed in polystyrene microplates and kept overnight at 4oC. After incubation and washing in PBS, nonspecific binding was blocked by adding 200µL of 10% ovalbumin (albumin from chicken egg whites grade II, Sigma-Aldrich, St Louis, USA) per well for 2 h at room temperature. After another wash, 100µL of hBD-1 mouse monoclonal antibody (IgG M4-14b-H4 -Santa Cruz, Dallas, USA) diluted 500 times in 10% ovalbumin was added per well, followed by two-hour incubation at room temperature. After another wash, 100µL of goat anti-mouse IgG-HRP (Santa Cruz, Dallas, USA) diluted 2,000 times in 10% ovalbumin was added to each well and then incubated for 45 minutes. After another wash, treatment with 2, 2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and ABTS enhancer (Sigma-Aldrich, St. Louis, USA) was performed according to the manufacturer’s recommendations to detect peroxidase activity. Absorbance was analyzed by an ELISA reader (DIATECH) at 405 nm. ELISA was also used for hBD-2 (hBD-2 ELISA Development Kit - Peprotech, New Jersey, USA) according to the manufacturer’s instructions. The saliva samples were diluted 1:2 in PBS and measured at 405 nm.
Statistical analysis
Prior to this study, the clinical parameters and salivary levels of hBD-1 and hBD-2 were determined in five patients from each of the three groups. The results of this pilot study were assessed by Student’s t test in order to calculate the sample size, using a 95% confidence interval and statistical power of 90%. The sample should include 35 patients per group, and we added 10% to this number, totaling 40 participants in each group. The data obtained from the pilot study were not accounted for in the final statistical analysis.
The data are presented as mean±standard deviation. The chi-square test was used to compare gender and number of smokers in the three groups. The Kolmogorov-Smirnov test was used to verify the normality of data distribution. Outliers were not removed from the data analysis. The Kruskal-Wallis test was used for analysis of three different means, followed by Dunn’s post-hoc test for assessment of differences between the means obtained for each group. In all cases, a 95% confidence interval was computed and statistical significance was obtained when P value<0.05. All statistical analyses were performed using the GraphPad software (San Diego, CA, USA).
A total of 120 patients (84 women and 36 men) aged 18 to 63 years were assessed as to their periodontal status using the PSR/CPITN index and as to their salivary levels of hBD-1 and hBD-2. The demographic data and the clinical parameters are summarized in Table 1. The mean age was similar between Groups 1 and 2; however, patients from Group 3 were older than those from Groups 1 and 2 (P<0.001). Also, Group 3 had a lower average number of teeth than Groups 1 and 2 (P<0.001).
|
Group 1 |
Group 2 |
Group 3 |
P |
Number |
40 |
40 |
40 |
- |
Age (years) |
26.7±8.1 |
33.4±13.7 |
43±11.2 |
< .0001* |
Males (%) |
25 |
27,5 |
37,5 |
0.4346 |
Smoking (%) |
10 |
7,5 |
17,5 |
0.3101 |
Mean number of teeth |
27.8±1.8 |
27.3±1.1 |
25.3±3.6 |
<0.0001* |
Sextant healthy - PSR code 0 |
6 |
2.1±1.4 |
0.4±0.7 |
<0.0001* |
Sextant BOP - PSR code 1 |
0 |
3.4 ±1.6 |
0.1±0.5 |
<0.0001* |
Sextant dental calculus - PSR code 2 |
0 |
0.5±0.7 |
0.9±1.4 |
<0.0001* |
Sextant PD 3.5 to 5.5 mm - PSR code 3 |
0 |
0 |
2.3±1.5 |
<0.0001* |
Sextant PD >5.5 mm - PSR code 4 |
0 |
0 |
2.1±1.9 |
<0.0001* |
Table 1 Demographic data and clinical parameters of three groups of patients according to their periodontal status, determined by the PSR index
*Statistically significant for Kruskal-Wallis one-way analysis of variance when compared to the three groups
BOP, bleeding on probing;
Group 1, periodontally healthy patients with all sextants classified as code 0;
Group 2, patients with at least one sextant scored as code 1 or 2;
Group 3, patients with at least one sextant scored as code 3 or 4.
Patients with periodontal disease (Groups 2 and 3) had a higher prevalence of BOP and higher pd than those with no periodontal disease (Group 1) (P<0.0001). No differences in smoking habits were found between the groups (P=0.31). Higher salivary levels of hBD-1 and hBD-2 were found in patients with periodontal disease. Patients with a PSR/CPITN index of 1 and 2 (7,750±3,477 pg/mL) and those with a PSR/CPITN index of 3 and 4 (7,363±2,837 pg/mL) had higher concentrations of hBD-1 in their saliva compared to periodontally healthy individuals (5,644±2,747 pg/mL). (P=0.013; Kruskal-Wallis test). Dunn’s post-hoc test showed some differences between Groups 1 and 2 and between Groups 1 and 3, but no difference was found for hBD-1 levels between Groups 2 and 3 (Figure 1).
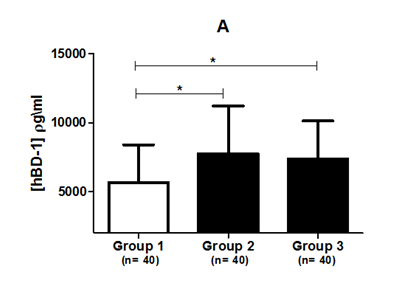
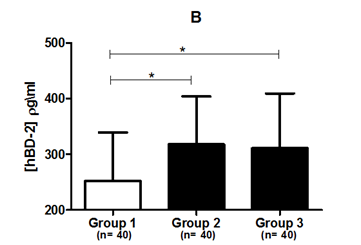
Figure 1 (A) Salivary hBD-1 concentration. (B) Salivary hBD-2 concentration in three different groups based on their periodontal status.
Group 1, periodontally healthy patients whose six sextants were classified as PSR code 0; Group 2, patients with at least one sextant scored as PSR code 1 or 2; Group 3, patients with at least one sextant scored as PSR code 3 or 4.
*Statistical significance in relation to periodontally healthy subjects using Dunn’s post-hoc test (P<0.05).
hBD-1, human beta-defensin 1; hBD-2, human beta-defensin 2; n, number of experiments.
As with hBD-1, salivary levels of hBD-2 were higher in Group 2 (317±85 pg/mL) and in Group 3 (310±98 pg/mL) compared to periodontally healthy subjects (251±87 pg/mL) (P=0.011; Kruskal-Wallis test). Dunn’s post-hoc test showed some difference in salivary levels of hBD-2 between periodontally healthy individuals and Group 2 and between periodontally healthy individuals and Group 3 (P<0.05); however, no difference was observed between Groups 2 and 3, i.e., between patients with periodontal disease (Figure 1).
A total of 120 patients (84 women and 36 men) aged 18 to 63 years were assessed as to their periodontal status using the PSR/CPITN index and as to their salivary levels of hBD-1 and hBD-2. The demographic data and the clinical parameters are summarized in Table 1. The mean age was similar between Groups 1 and 2; however, patients from Group 3 were older than those from Groups 1 and 2 (P<0.001). Also, Group 3 had a lower average number of teeth than Groups 1 and 2 (P<0.001).
Patients with periodontal disease (Groups 2 and 3) had a higher prevalence of BOP and higher pd than those with no periodontal disease (Group 1) (P<0.0001). No differences in smoking habits were found between the groups (P=0.31). Higher salivary levels of hBD-1 and hBD-2 were found in patients with periodontal disease. Patients with a PSR/CPITN index of 1 and 2 (7,750±3,477 pg/mL) and those with a PSR/CPITN index of 3 and 4 (7,363±2,837 pg/mL) had higher concentrations of hBD-1 in their saliva compared to periodontally healthy individuals (5,644±2,747 pg/mL). (P=0.013; Kruskal-Wallis test). Dunn’s post-hoc test showed some differences between Groups 1 and 2 and between Groups 1 and 3, but no difference was found for hBD-1 levels between Groups 2 and 3 (Figure 1).
As with hBD-1, salivary levels of hBD-2 were higher in Group 2 (317±85 pg/mL) and in Group 3 (310±98 pg/mL) compared to periodontally healthy subjects (251±87 pg/mL) (P=0.011; Kruskal-Wallis test). Dunn’s post-hoc test showed some difference in salivary levels of hBD-2 between periodontally healthy individuals and Group 2 and between periodontally healthy individuals and Group 3 (P<0.05); however, no difference was observed between Groups 2 and 3, i.e., between patients with periodontal disease (Figure 1).
Many researchers have sought to identify for biomarkers of periodontal disease to monitor its progression or facilitate its diagnosis. This study evaluated h-BD-1 and h-BD2 salivary as a possible marker of periodontal disease. Periodontal disease presents a long time consuming and expensive treatment, therefore its prevention, early detection and management are issues which, if effectively addressed, are likely to yield considerable health-care benefit.6 The presence of saliva is critical for the preservation and maintenance of the oral tissues, and it has also been used as a source of non-invasive investigation. Thus, it could contain important biomarkers related to physiological aspects of periodontal diseases, which means that qualitative changes in biomarkers composition might have diagnostic and therapeutic significance.18 Early studies focused on serum and gingival crevicular fluid samples for investigations of periodontitis related cytokines; however, in recent years, saliva has become an alternative source of biomarkers. The saliva collection has several advantages over gingival crevicular fluid: it is more easily accessible; it can be sampled in a much larger volume without the need for clinical facilities; and no complex skills are necessary for sampling. Furthermore, whereas the gingival crevicular fluid content reflects inflammatory processes at individual disease sites, it is reasonable to suggest that saliva content reflects a ‘whole mouth’ inflammatory status, which is likely to be much more clinically relevant.6
Studies investigating the use of saliva as a diagnostic fluid have a long history. This noninvasive approach is not limited only to the diagnosis of oral diseases because many systemic diseases, such as different types of cancers, cardiovascular diseases, immunologic syndromes and hereditary deficiencies, can also be studied with the aid of salivary diagnostics (3,20,38,110). The continuous interaction between host cells and pathogenic bacteria are the hallmark of the development of periodontal disease. Gingival epithelial cells, mostly keratinocytes, act not only as a physical barrier against microbial factors, but also mediate the immune response by secreting antimicrobial peptides (e.g., hBD).19,20 In general, hBD-1 is continuously produced by keratinocytes,21,22 but some studies have suggested that hBD-1 production can be stimulated by bacterial products and inflammatory mediators.23–25 In the present study, hBD-1 expression was observed in all samples, but patients with periodontal disease (Groups 2 and 3) had higher hBD-1 levels in their saliva when compared to their periodontally healthy counterparts (Group 1). According to Matheus et al.26 continuous contact of the oral mucosa oral with the commensal microflora can stimulate hBD-1 production even in the absence of periodontal disease; however, the presence of pathogenic microorganisms and the presence of an inflammatory process could increase hBD-1 levels. The present study did not indicate an increase in salivary levels of hBD-1 with the deterioration of periodontal disease as these levels were similar between patients with a PSR/CPITN index of 1 and 2 (Group 2) and between those with a PSR/CPITN index of 3 and 4 (Group 3); nevertheless, h-BD1 levels indicated the presence of periodontal disease since h-BD1 levels in the saliva of periodontally healthy patients (Group 1) were lower than those observed among patients with periodontal disease (Groups 2 and 3).
Regarding hBD-2 levels, this study revealed they were similar to those obtained for hBD-1. Higher concentrations were observed in Groups 2 and 3 when compared to Group 1. Albeit nonstatistically significant, hBD-1 and hBD-2 levels were slightly lower in patients with a PSR/CPITN index of 3 and 4 when compared to those with a PSR/CPITN index of 1 and 2. We believe this small reduction in hBD-1 and hBD-2 levels in the saliva occurred due to the degradation of hBD by proteases released at sites that are more commonly affected by periodontopathogens, as hypothesized by Yong et al.27 who verified higher hBD-2 levels in the saliva of patients with gingivitis than among those with periodontitis. Another possible explanation to this reduction could be the lower average number of teeth in Group 3.
The quantification of hBD-1 and hBD-2 by ELISA has been widely used for the determination of salivary levels of these peptides.14,26 There are conflicting reports in the current literature regarding hBD-1 and hBD-2 expression in periodontal disease, as some studies suggest upregulated hBD expression8,14,27,28 whereas some suggest a down regulated expression of this peptide.29,30 In addition, previous studies found high concentrations of these peptides only after stimulation with periodonto pathogens.31,32 The difference in salivary levels of hBD between this and other studies could be related to the method used in the present one since the mRNA expression of hBDs measured by real-time PCR might not indicate the actual concentrations of salivary peptides.33
Owing to the higher salivary levels of hBD-1 and hBD2 detected by ELISA in patients with periodontal disease, these antimicrobial peptides could act as potential biomarkers of periodontal disease. However, although individual salivary hBD-1 and hBD-2 levels can be statistically different between the samples obtained from periodontally healthy and periodontally compromised patients, the large variation in individual levels could prove a great hindrance to its use as biomarker of periodontal disease.5 A possible solution would be the use of diagnostic panels that combine different biomarkers.34
The lack of microbiological investigation does not allow us to determine the relationship between hBD levels and the presence of periodontopathogens and its role in the pathogenesis of periodontal disease. In conclusion, salivary levels of hBD-1 and hBD-2 were high in groups with periodontal disease detected by PSR/CPITN index when compared to the healthy group. However, the concentrations of these defensins did not vary as a result of the deterioration of periodontal condition. hBD-1 and 2 are possible candidates as periodontal disease markers, but they cannot indicate inflammatory periodontal stages.
Lourenço AG and Komesu MC conceived the research; Ribeiro AERA recruited the patients and provided the samples; Lourenço AG and Ribeiro AERA performed the experiments; Lourenço AG, Komesu MC, Motta ACF and Ribeiro AERA analyzed the data; Lourenço AG, Komesu MC, Motta and Ribeiro AERA wrote the paper.
Author declared there is no conflict of interest.

©2018 Ribeiro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.