Journal of
eISSN: 2373-437X


Research Article Volume 3 Issue 3
1Department of Microbiology and Immunology, Chicago College of Osteopathic Medicine, Midwestern University, USA
2Division of Infectious Diseases, St. John Providence Health System, USA
3Department of Obstetrics and Gynecology, St. John Providence Health System, USA
4Division of Infectious Diseases, Henry Ford Health System, USA
5Jose A. Vazquez, M.D., Division of Infectious Diseases, Medical College of Georgia/Georgia Regents University, USA
Correspondence: Melphine M Harriott, Ph.D., Department of Microbiology and Immunology, Chicago College of Osteopathic Medicine, Midwestern University, 555 31st Street, Downers Grove, IL 60515, USA, Tel 630-515-7428, Fax 630-515-7245
Received: March 01, 2016 | Published: April 11, 2016
Citation: Harriott MM, Shoyinka AT, Ponniah MP, Doyon-Reale N, Vager D, et al. (2016) In Vitro Antifungal-Antibacterial Combinations are Effective Against MRSA in Candida albicans-Staphylococcus aureus Polymicrobial Biofilms. J Microbiol Exp 3(3): 00090. DOI: 10.15406/jmen.2016.03.00090
A significant proportion of human infections involve biofilms, which are more resistant to antimicrobial agents than their planktonic counterparts. It is well established that C. albicans readily forms polymicrobial biofilms with S. aureus. We hypothesized that the addition of an antifungal to an antibacterial, or combination therapy would be more effective in reducing the microbial load, most notably in polymicrobial biofilms, and possibly impact overall management of infection. In vitro results demonstrate that clinical strains of both methicillin-susceptible and methicillin-resistant S. aureus are less susceptible to vancomycin in polymicrobial biofilms with C. albicans when compared to S. aureus monomicrobial biofilms. Treatment of biofilms with either amphotericin B or caspofungin in combination with vancomycin resulted in greater inhibition and even elimination of methicillin-susceptible and methicillin-resistant S. aureus in C. albicans polymicrobial biofilms. The results of this study indicate that combination therapy should be a consideration in the management of polymicrobial biofilms, specifically C. albicans-S. aureus biofilm infections.
Keywords: C. albicans, S. aureus, polymicrobial biofilms, antimicrobial susceptibility
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; H, hour
Biofilms are a significant contributing factor to human infections and medically important biofilms frequently develop on medical devices such as intravascular catheters, often resulting in severe and potentially fatal catheter-related-bloodstream infections (CRBSIs).1,2 In the United States, approximately 250,000 cases of CRBSIs occur annually, increasing not only health care cost but also morbidity and mortality.3-5 Two common pathogens involved in CRBSIs are Candida albicans and Staphylococcus aureus.6
One of the complicating factors of many infections, including intravascular catheter related infections, is the presence of multiple organisms. In the past, organisms isolated in low quantities from polymicrobial cultures were often considered contaminants and disregarded from the antimicrobial therapy plan. Increasing evidence suggests that microorganisms within the polymicrobial milieu, especially within a biofilm, influence each other, leading to increases in pathogenicity or resistance of one or more of the organisms.7-10 Increased resistance may then lead to prolonged illnesses and higher morbidity and mortality. Prior research has demonstrated that S. aureus forms poor monomicrobial biofilms in serum, but a robust polymicrobial biofilm with C. albicans.11-14 Furthermore, methicillin-sensitive S. aureus (MSSA) in the C. albicans polymicrobial biofilm exhibits decreased susceptibility to vancomycin when compared to S. aureus monomicrobial biofilms.12 However, simultaneous exposure of these biofilms to amphotericin B and vancomycin abrogates the decreased susceptibility of S. aureus to vancomycin. This has enormous clinical implications as S. aureus is the third most common organism isolated with C. albicans in bloodstream infections.6,15
It is well established that organisms within a biofilm are more resistant to antimicrobials and are protected from the host immune response when compared to their planktonic counterparts. Therefore, antimicrobial therapy options to eliminate biofilm-associated infections are urgently needed. Unfortunately, current clinical microbiology protocols only examine the susceptibility patterns of planktonic organisms. Moreover if the infection is polymicrobial, combination antifungal-antibacterial therapy may be needed to improve the clinical outcome. The two most common antimicrobials that are utilized for CRBSIs are the echinocandins, such as caspofungin, for the treatment of Candida and vancomycin for the treatment of S. aureus, specifically MRSA.4 MRSA continues to play a significant role in CRBSIs in the United States where over half of the device-associated infections with S. aureus are resistant to methicillin.6,16,17 The effects of combination antimicrobials on methicillin-resistance S. aureus (MRSA) are unknown.
In this study we evaluated antifungal and antibacterial combinations of amphotericin B or caspofungin with vancomycin on C. albicans-MSSA or C. albicans-MRSA polymicrobial biofilms. We hypothesized that combination antimicrobials would be more beneficial for reducing biofilm viability and eradicating microorganisms than single-agents. We also evaluated whether clinical strains of MSSA and MRSA, like laboratory strains, demonstrated increased tolerance to vancomycin in a C. albicans S. aureus polymicrobial biofilm.
C. albicansstrains and handling
The clinical strain of C. albicans, HFH-1, used in this study was obtained from Henry Ford Hospital and Medical Center (HFH). The strain was confirmed as C. albicans by the HFH clinical microbiology laboratory. For each experiment, the organism was subcultured from freezer stocks on Sabouraud dextrose agar (SDA) plates and incubated overnight at 30°C. Subsequent liquid cultures were prepared from colonies derived from these plates. Liquid cultures were prepared by incubating C. albicans colonies in Sabouraud Dextrose Broth (SDB) overnight at 30°C in an orbital shaker (200rpm).
S. aureusstrain and handling
S. aureusclinical isolates MSSA HFH-1 and MRSA HFH-1 were obtained from Henry Ford Hospital and Medical Center (HFH) and were confirmed by the HFH clinical microbiology laboratory as S. aureus. Strains were subcultured from freezer stocks onto brain heart infusion (BHI) agar plates for each experiment and incubated at 35°C overnight. Subsequent liquid subcultures were derived from colonies isolated from these plates and were prepared by incubating S. aureus, MSSA or MRSA in BHI broth overnight at 35°C in an orbital shaker (200rpm).
Biofilm formation
Overnight cultures were generated as described above. Cultures were then washed twice in sterile 1X phosphate-buffered saline (PBS) and resuspended in 1X PBS. Biofilms were formed in 50% bovine serum (BS) as previously described.12,14 The final concentrations of organisms were 106 CFU/mL of C. albicans and 107 CFU/mL of S. aureus. Control wells contained C. albicans alone, S. aureus alone or 50% BS alone in wells of flat-bottom 96-well polystyrene plates.
Biofilm antimicrobial assay
After a 24h incubation at 35°C, biofilm plates were gently washed three times with 1X PBS to remove nonadherent cells. Stock solutions of amphotericin B (Fisher Scientific, Hanover, IL), caspofungin (Merck KGaA, Darmstadt, Germany) and vancomycin (Akorn-Strides, Lake Forest, IL) were prepared in dimethyl sulfoxide (DMSO) and diluted to working concentrations in RPMI (Sigma-Aldrich, St. Louis, MO). The final concentration of DMSO was ≤1%. Antimicrobials alone or in combination were added to the washed biofilms and RPMI was added as needed for a final well volume of 100μl. We utilized RPMI for antimicrobial testing because of the possibility that serum factors may bind to certain antimicrobials.18 Control wells contained no antimicrobials but did contain ≤1% DMSO. The plates were incubated for 24 h and fungal, and bacterial viability was monitored by the CFU assay.
Planktonic antimicrobial assay
Planktonic susceptibilities were performed with a modification of the CLSI standards for broth dilution. C. albicans (103) and S. aureus (103) alone or in combination were added to 96 deep-well polypropylene plates (Fisher Scientific, Hanover IL) in RPMI. Bacterial and fungal suspensions were mixed in the wells by pipetting. Antimicrobials alone or in combination were then added to the wells. The final well volume was adjusted with RPMI to 200μl. The plates were incubated at 35°C in an orbital shaker at 175rpm for 18h and fungal and bacterial viability was monitored by the CFU assay.
CFU assay
The CFU assay was performed as previously described [12,14] and tenfold dilutions of each well were made and plated on SDA supplemented with ampicillin (0.008mg/mL) and erythromycin (0.075mg/mL) and also on BHI agar supplemented with amphotericin B (0.025mg/mL). Plates were then incubated at 35°C for 24 h and colonies were counted and recorded.
Significance
For this study any changes in susceptibility that resulted in a ≥1 log CFU/mL change was considered significant.
Single-agent vancomycin is unable to inhibit clinical strains of MSSA or MRSA in polymicrobial biofilms with C. albicans
Although vancomycin was developed over 50 years ago, it was infrequently used clinically until the emergence of MRSA in the 1980s.19 At the present, vancomycin remains the antimicrobial of choice for MRSA infections. Because previous studies evaluated laboratory strains of MSSA, we initially evaluated the activity of vancomycin against clinical strains of MSSA to confirm a decrease in susceptibility to vancomycin when part of a C. albicans polymicrobial biofilm. Additionally, we wanted to determine the effect of vancomycin on clinical strains of MRSA in monomicrobial and C. albicans-MRSA polymicrobial biofilms. To ensure that susceptibility patterns were biofilm-specific, we also tested monomicrobial and polymicrobial C. albicans, MSSA and MRSA using planktonic methodology.
Monomicrobial biofilms were formed as described in the methods with either MSSA or MRSA alone. After a 24h incubation at 35°C, vancomycin was added to the washed biofilms. After an additional 24h at 35°C, plates were washed, and CFUs were analyzed. Planktonic susceptibilities were formed using C. albicans alone, MSSA alone, MRSA alone or the combination of C. albicans-MSSA or C. albicans-MRSA together in 96-well round bottom plates as described in the methods. Vancomycin was added to the plates, and the plates were incubated in an orbital shaker at 35°C to prevent biofilm formation for 18h. Viability was monitored with the CFU assay.
As shown in prior publications using laboratory strains of MSSA,12 the clinical strain of MSSA in this study also exhibits a decrease in susceptibility to vancomycin in the presence of C. albicans (Figure 1a). Even at the highest dose tested, 1000μg/mL of vancomycin, MSSA in the polymicrobial biofilm is not affected. In contrast, MSSA in a monomicrobial biofilm is inhibited by vancomycin (Figure 1a). In the MSSA monomicrobial biofilm, 7.81μg/mL of vancomycin reduces the viability by approximately 2-logs, and at the highest concentration of 1000μg/mL, there is a 5-log reduction in CFUs (Figure 1a).
Additionally, MRSA in the polymicrobial biofilm is also less susceptible to vancomycin, whereas MRSA in monomicrobial biofilms is inhibited by vancomycin (Figure 1b). There is an approximate 2-log reduction in CFUs with 1.95μg/mL of vancomycin and a 3-log reduction in monomicrobial MRSA CFUs with 250μg/mL, which is the maximum reduction in viability observed. This also suggests that MRSA in the monomicrobial biofilm is less susceptible to vancomycin than MSSA in the monomicrobial biofilm (Figure 1a and Figure 1b). Conversely, MRSA in a C. albicans-MRSA polymicrobial biofilm is not affected by vancomycin (Figure 1b). As might be expected, vancomycin alone has no effect on C. albicans in either the polymicrobial or monomicrobial biofilm or planktonic cultures (Figure 1a-d).

Figure 1 Effect of vancomycin alone on biofilms and planktonic cultures. Biofilms were formed in 50% bovine serum using C. albicans HFH-1 (106 CFU/mL) and a) methicillin-sensitive S. aureus HFH-1 (107 CFU/mL) or b) methicillin-resistant S. aureus (107 CFU/mL) alone or together. Organisms were added to tissue culture treated 96-well plates and plates were incubated for 24h at 35°C. After 24h, vancomycin in RPMI was added to washed plates. After an additional incubation for 18 h at 35°C, CFUs were determined by plating onto selective media. For planktonic susceptibilities, C. albicans HFH-1 (103 CFU/mL) alone or with c) methicillin-sensitive S. aureus HFH-1 (103 CFU/mL) or d) methicillin-resistant S. aureus (103 CFU/mL) were added to 96-well, deep well polypropylene plates in RPMI. Vancomycin was then added to the wells. Plates were incubated at 35°C in an orbital shaker at 175rpm for 18h, and fungal and bacterial viability was monitored by CFU assay. Experiments were performed in duplicate and are representative of 3 independent experiments. CA, C. albicans; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; Mono, monomicrobial; Poly, polymicrobial.
This decrease in susceptibility to vancomycin appears to be biofilm-specific since vancomycin inhibits MSSA and MRSA in both planktonic monomicrobial and polymicrobial cultures in a similar manner (Figure 1c & 1d). Biofilm and planktonic susceptibility patterns are not strain specific since we also examined other combinations of clinical and laboratory strains of C. albicans, MSSA and MRSA and all demonstrated similar susceptibility profiles as seen in this study (results not shown). This data confirms that clinical strains of MSSA and MRSA in C. albicans polymicrobial biofilms, but not in monomicrobial and polymicrobial biofilms or planktonic cultures, are less susceptible to vancomycin.
Preliminary data in our laboratory reveals that MSSA and MRSA in C. albicans polymicrobial biofilms appear to be more tolerant to other antibacterial agents, including linezolid, daptomycin, cephalexin, and ceftaroline when compared to monomicrobial MSSA and MSSA biofilms (results not shown). This provides further evidence of decreased antimicrobial susceptibility when C. albicans and S. aureus are found in polymicrobial biofilms and thus the need to further explore antimicrobial combination as a viable option in polymicrobial infections.
Combination antibacterial and antifungal treatment is effective in inhibiting MSSA or MRSA in polymicrobial biofilms with C. albicans
First line antifungal agents for candidemia or invasive candidiasis includes azoles such as fluconazole, or echinocandins, such as caspofungin.20 Echinocandins, which inhibit cell wall synthesis by targeting β-1,3-glucan, are favored over fluconazole due to increases in fluconazole resistance in some Candida species.21 However, if these first-line antifungals fail to treat these infections, amphotericin B, a polyene, that binds to ergosterol may also be used.
Previous research demonstrated that while laboratory strains of MSSA in polymicrobial biofilms do not respond to a single-agent, such as vancomycin, simultaneous exposure of biofilms to amphotericin B and vancomycin did reduce the MSSA viability.12 Therefore, we assessed whether treatment with an antifungal (amphotericin B or caspofungin) in combination with vancomycin would also reduce or eradicate clinical strains of MSSA or MRSA in C. albicans-MSSA or C. albicans-MRSA polymicrobial biofilms. Because previous work evaluated combination effects of amphotericin B and vancomycin on C. albicans-MSSA, we assessed amphotericin B and vancomycin on C. albicans-MRSA biofilms.
Monomicrobial or polymicrobial biofilms were formed as previously described with either MSSA or MRSA alone or in combination with C. albicans. After a 24h incubation at 35°C, vancomycin alone, antifungals alone, or vancomycin and the antifungals together were added to the washed biofilms. After 24h at 35°C, plates were washed, and CFUs were analyzed.
Effect of amphotericin B + vancomycin on MRSA biofilms
We evaluated the effect of amphotericin B alone or in combination with vancomycin on monomicrobial and polymicrobial C. albicans-MRSA biofilms (Figure 2a & 2b). Amphotericin B alone is fungicidal for C. albicans in both the monomicrobial and polymicrobial biofilms (Figure 2a). However, the minimum fungicidal concentration (MFC) is greater for C. albicans in the polymicrobial biofilm, when compared to the monomicrobial biofilm (15.63μg/mL vs. 3.91μg/mL) (Figure 2a). As anticipated, amphotericin B alone has no effect on MRSA, in either the monomicrobial or polymicrobial biofilm (Figure 2a).
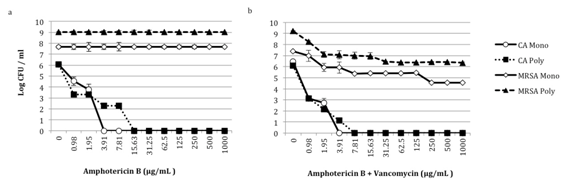
Figure 2 Effect of amphotericin B alone and with vancomycin on C. albicans-MRSA biofilms. Biofilms were formed in 50% bovine serum using C. albicans HFH-1 (106 CFU/mL) and methicillin resistant S. aureus HFH-1 (107 CFU/mL) alone or together. Organisms were added to tissue culture treated 96-well plates and plates were incubated for 24 h at 35°C. After 24 hours, a) amphotericin B or b) amphotericin B and vancomycin in RPMI was added to washed plates. After an additional incubation for 18 h at 35°C, CFUs were determined by plating onto selective media. Experiments were performed in duplicate and are representative of 3 independent experiments. CA, C. albicans; MRSA, methicillin-resistant S. aureus; Mono, monomicrobial; Poly, polymicrobial.
Biofilms were also exposed to amphotericin B and vancomycin simultaneously. MRSA in the polymicrobial biofilm becomes susceptible to vancomycin, and the decrease in MRSA CFUs corresponds to a decrease in C. albicans viability (Figure 2b). With 0.98μg/mL of amphotericin B and 0.98μg/mL of vancomycin, there is approximately a 3-log reduction in C. albicans in the polymicrobial biofilm. At this same antimicrobial concentration, MRSA in the polymicrobial biofilm demonstrates about a 1-log reduction in CFUs. When the concentration of amphotericin B and vancomycin is increased to 1.95μg/mL, there is approximately a 4-log reduction in C. albicans and a 2-log decrease in MRSA CFUs (Figure 2b). This is in stark contrast to the lack of MRSA inhibition observed when polymicrobial biofilms are exposed to vancomycin alone (Figure 1b). MRSA monomicrobial biofilms demonstrate a similar level of susceptibility when treated with vancomycin alone, compared with combination amphotericin B and vancomycin (Figure 1b & 2b). Additionally, there is no significant difference in susceptibility patterns for C. albicans in monomicrobial biofilms or in C. albicans-MRSA polymicrobial biofilms. This data confirms that MRSA in polymicrobial biofilms is less susceptible to vancomycin, and that the combination of amphotericin B and vancomycin appears to be more effective than vancomycin alone against MRSA in a polymicrobial biofilm.
Effect of caspofungin + vancomycin on MSSA and MRSA biofilms
Caspofungin and vancomycin alone and in combination were also evaluated in both monomicrobial and polymicrobial C. albicans-MSSA (Figure 3a & 3b) and C. albicans-MRSA (Figure 4a & 4b) biofilms.

Figure 3 Effect of caspofungin alone and with vancomycin on C. albicans-MSSA biofilms. Biofilms were formed in 50% bovine serum using C. albicans HFH-1 (106 CFU/mL) and methicillin-sensitive S. aureus HFH-1 (107 CFU/mL) alone or together. Organisms were added to tissue culture treated 96-well plates and plates were incubated for 24h at 35°C. After 24h, a) caspofungin or b) caspofungin and vancomycin in RPMI was added to washed plates. After an additional incubation for 18h at 35°C, CFUs were determined by plating onto selective media. Experiments were performed in duplicate and are representative of 3 independent experiments. CA, C. albicans; MSSA, methicillin-sensitive S. aureus; Mono, monomicrobial; Poly, polymicrobial.
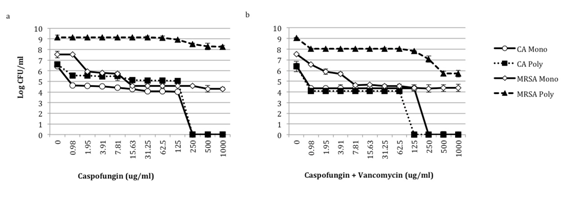
Figure 4 Effect of caspofungin alone and with vancomycin on C. albicans-MRSA Biofilms. Biofilms were formed in 50% bovine serum using C. albicans HFH-1 (106 CFU/mL) and methicillin-resistant S. aureus HFH-1 (107 CFU/mL) alone or together. Organisms were added to tissue culture treated 96-well plates and plates were incubated for 24 h at 35°C. After 24h, a) caspofungin or b) caspofungin and vancomycin in RPMI was added to washed plates. After an additional incubation for 18h at 35°C, CFUs were determined by plating onto selective media. Experiments were performed in duplicate and are representative of 3 independent experiments. CA, C. albicans; MRSA, methicillin-resistant S. aureus; Mono, monomicrobial; Poly, polymicrobial.
MSSA biofilms
Caspofungin alone displays fungicidal effects against C. albicans in monomicrobial biofilms (Figure 3a and 4a) and C. albicans in C. albicans-MSSA polymicrobial biofilms (Figure 3a). With 0.98μg/mL of caspofungin, C. albicans in the monomicrobial biofilms and C. albicans in the MSSA polymicrobial biofilm demonstrates about a 2-log decrease in CFUs. Furthermore, the addition of 250μg/mL of caspofungin results in elimination of C. albicans (Figure 3a) in the monomicrobial biofilm. Treatment of biofilms with caspofungin and vancomycin does not significantly alter the viability of C. albicans monomicrobial or polymicrobial biofilm when compared to caspofungin alone (Figure 3b).
Caspofungin alone has an inhibitory effect on MSSA and MRSA monomicrobial and polymicrobial biofilms (Figure 3a & 4a). With 250μg/mL of caspofungin MSSA monomicrobial biofilms demonstrate approximately a 3-log reduction in CFUs and with 1000μg/mL of caspofungin the viability is reduced by almost 5-logs (Figure 3a). This decrease in viability is similar to the inhibition observed when MSSA monomicrobial biofilms are treated with vancomycin alone (Figure 1a). Treatment of MSSA monomicrobial biofilms with 3.91μg/mL of caspofungin and vancomycin together results in a 3-log decrease in CFUs, while 62.5μg/mL of both agents results in nearly 100% bactericidal activity against MSSA in monomicrobial biofilms (Figure 3b).
In MSSA polymicrobial biofilms, ≥250μg/mL of caspofungin alone reduces the CFUs by 4-logs (Figure 3a). As with MSSA monomicrobial biofilms, the combination of caspofungin and vancomycin better reduces viability compared to monotherapy. With 0.98μg/mL of caspofungin and vancomycin, there is about a 1-log decrease in MSSA in the polymicrobial biofilm. This also corresponds to a 2-log decrease in C. albicans in the polymicrobial biofilm. Moreover, with 125μg/mL of caspofungin and vancomycin there is a 6-log decrease in MSSA in the polymicrobial biofilm and at concentrations above 250μg/mL, MSSA in the polymicrobial biofilm is eliminated (Figure 3b). In contrast, caspofungin alone or vancomycin alone is not as effective in reducing MSSA polymicrobial biofilm viability (Figure 1a & 3a).
MRSA biofilms
Similar to C. albicans-MSSA polymicrobial biofilms, C. albicans in the MRSA polymicrobial biofilm is also completely killed by caspofungin (Figure 4a). However, C. albicans in the presence of MRSA is more tolerant of caspofungin inhibition when compared to C. albicans in MSSA biofilms (Figure 3a & Figure 4a). In the biofilm with MSSA, C. albicans demonstrates a 2-log reduction in viability with 0.98-125μg/mL of caspofungin. In contrast, with the same caspofungin concentration range only a 1-log decrease in CFUs is noted for C. albicans in biofilms with MRSA (Figure 3a & 4a). Combination caspofungin and vancomycin did not significantly alter the susceptibility of C. albicans monomicrobial biofilms compared to caspofungin alone (Figure 4a & 4b).
As with MSSA, MRSA in the monomicrobial and in the C. albicans-polymicrobial biofilm is inhibited by caspofungin alone. In MRSA monomicrobial biofilms, 1.95μg/mL of caspofungin decreases CFUs by about 1.5-logs (Figure 4a). This is similar to the reduction in MRSA monomicrobial biofilms with 1.95μg/mL of vancomycin alone (Figure 1b). Furthermore, 15.63μg/mL of caspofungin alone reduces the MRSA monomicrobial biofilm viability by approximately 3-logs (Figure 4a). To reach this same decrease in biofilm viability of MRSA monomicrobial biofilms, ≥250μg/mL of vancomycin alone is necessary (Figure 1b). With 7.81μg/mL of vancomycin and caspofungin, a 3-log reduction in CFUs is observed (Figure 4b); whereas ≥250μg/mL of either vancomycin or ≥15.93μg/mL of caspofungin is required to reach this same decrease in viability (Figure 1b & 4a).
In contrast to MSSA and MRSA monomicrobial biofilms, MRSA in the polymicrobial biofilm demonstrates only a 0.5-log reduction in CFUs with 250μg/mL of caspofungin (Figure 4a). However, with 0.98μg/mL of caspofungin and vancomycin, a 1-log decrease in CFUs of MRSA in the polymicrobial biofilm is observed. This corresponds to a 2-log decrease in C. albicans in the polymicrobial biofilm. Caspofungin and vancomycin together at concentrations of ≥500μg/mL result in a 3-log reduction in MRSA CFUS in the polymicrobial biofilm (Figure 4b). This is significant since vancomycin alone does not reduce the viability of MRSA in the polymicrobial biofilm, even at 1000μg/mL (Figure 1b).
Taken together this data demonstrates that caspofungin alone appears to have some anti-staphylococci activity in biofilms, and the combination of caspofungin and vancomycin is better than monotherapy. The anti-staphylococci effects of caspofungin are not observed with planktonic monomicrobial or polymicrobial cultures (Figure 51a-51d); however the effect was still noted with biofilms when other strains of MSSA or MRSA were used (results not shown). The mechanisms mediating the antibacterial effect of caspofungin are unknown and may be multifactorial. There is also the possibility that characteristics of S. aureus, such as the capsule or slime production may play a role in the ability of the bacteria to respond to caspofungin. In the future, we plan on investigating other echinocandin and antibacterial combinations to see if similar susceptibility patterns are noted.
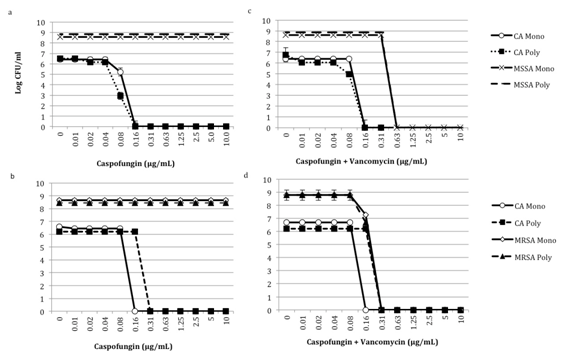
Figure 5 Effect of caspofungin alone and with vancomycin on C. albicans-MSSA and MRSA planktonic cultures. C. albicans HFH-1 (103 CFU/mL) alone or with Panels a and b) methicillin-sensitive S. aureus HFH-1 (103 CFU/mL) or c and d) methicillin-resistant S. aureus (103 CFU/mL) were added to 96-well, deep well polypropylene plates in RPMI. a and b) Caspofungin alone or c and d) in combination with vancomycin was then added to the wells. Plates were incubated at 35°C in an orbital shaker at 175rpm for 18h, and fungal and bacterial viability was monitored by CFU assay. Experiments were performed in duplicate and are representative of 3 independent experiments. CA, C. albicans; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; Mono, monomicrobial; Poly, polymicrobial.
Future work will be required to determine if the effect of the antifungal and antibacterial combinations examined in this study are synergistic or additive. Preliminary work in our laboratory shows that other combinations of antifungals and vancomycin or other anti-staphylococcal agents also inhibit or kill MSSA or MRSA in polymicrobial biofilms with C. albicans. Additionally, this does not appear to be strain specific since we have tested other clinical strains of C. albicans, MSSA and MRSA in various combinations and the results remain consistent.
The concentrations used in this study are higher than what could be utilized clinically. In order to determine which combinations could be clinically beneficial, future work using an in vivo murine model will further investigate these combinations at more clinically relevant concentrations. Additionally, the question of what is mediating the antibacterial resistance of S. aureus in the C. albicans polymicrobial biofilm must further be explored. The C. albicans matrix plays a minor role in enhanced drug tolerance;12 however, other mechanisms including C. albicans mannans or glucans could be impacting the S. aureus response to vancomycin.
In this study, we confirm that clinical strains of MSSA or MRSA in polymicrobial biofilms are more resistant to vancomycin. We also established that combination antibacterial and antifungal therapy is beneficial in reducing or even eradicating MSSA and MRSA in polymicrobial biofilms.
Both C. albicans and S. aureus are normal inhabitants of the skin and frequently colonize catheters forming complex biofilms, which may ultimately result in life-threatening CRBSIs. Catheter removal and antimicrobial adminsistration are recommended, however in many of these device-related infections the device cannot be removed for a variety of reasons. In these cases, an aggressive yet non-invasive therapeutic strategy, such as combination antimicrobial therapy for the management of polymicrobial biofilm infections would greatly benefit the patient. Unfortunately, in routine clinical practice, planktonic MICs and not biofilm MICs are provided through the clinical laboratory and thus do not reflect the true susceptibility profile of biofilm-associated pathogens. Furthermore, as demonstrated in this study, organisms within the polymicrobial biofilm may frequently influence the susceptibility of other organisms, leading to secondary resistance and increased clinical failure rates despite apparently susceptible organisms.
In conclusion, the results of this study suggest that combination antifungal and antibacterial therapy appears to be more effective in reducing and possibly even eradicating infections due to polymicrobial biofilms than monomicrobial therapy. Future work will focus on determining the most appropriate antifungal and antibacterial combinations to reduce C. albicans and S. aureus in polymicrobial biofilms and to determine if these combinations are also effective in vivo.
None.
Authors declare there are no conflicts of interest.

©2016 Harriott, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.