Journal of
eISSN: 2373-437X


Mini Review Volume 5 Issue 1
1Department of Internal Medicine, University of Cyprus Medical School, Cyprus
2Department of Internal Medicine, Patras University General Hospital, Greece
3Center for Cancer Research, National Cancer Institute, USA
4Department of Microbiology, Korgialenio-Benakio Red Cross Hospital, Greece
5Department of Internal Medicine, University of Cyprus Medical School, Cyprus
Correspondence: Antreas Ioannou, Nicosia General Hospital, Department of Internal Medicine, University of Cyprus Medical School, 2029 Strovolos, Nicosia, Cyprus, Tel 35722603447, Fax 35722604322
Received: May 15, 2017 | Published: June 1, 2017
Citation: Ioannou A, Andreou M, Watson DC, Chra P, Panos G (2017) Hantavirus Infections in the European Region: A Mini-Review of the Literature. J Microbiol Exp 5(1): 00139. DOI: 10.15406/jmen.2017.05.00139
Hantaviruses, members of the Bunyaviridae family, are emerging zoonotic infectious agents causing Haemorrhagic Fever with Renal Syndrome (HFRS) in Europe and Asia, and Hantavirus Pulmonary Syndrome (HPS) in the Americas. Humans acquire infection after inhalation of contaminated aerosolized excreta of infected rodents. The trend towards increasing cases reported in the European region suggests a need for clinicians to be vigilant for the diagnosis of such infections.
Keywords: Viral hemorrhagic fever; Clinical; Pathophysiology; Hemorrhagic fever with renal syndrome; Hantavirus pulmonary syndrome
HFRS, haemorrhagic fever with renal syndrome; HPS, hantavirus pulmonary syndrome; ICU, intensive care unit; VEGF, vascular endothelial growth factor; CSF, cerebrospinal fluid; DIC, disseminated intravascular coagulation; CRP, C-reactive protein; HRCT, high-resolution computed tomography; PCR, polymerase chain reaction; ECMO, extracorporeal membrane oxygenation
Hantavirus is a relatively newly discovered virus, with worldwide distribution, isolated in 1976 by Ho-Wang Lee (South Korean Virologist) & Karl M. Johnson (American tropical virologist).1 The first infections were identified years after, an outbreak of Korean haemorrhagic fever among soldiers during the Korean War in the early 1950s.1 Each Hantavirus species is associated with a specific rodent or insectivore host in each geographic region and only a portion of them are pathogenic in humans.2 Human infection is caused after inhalation of aerosolized host-reservoir excreta or body fluids (saliva or urine), contaminated with replication competent virus particles.3 Human to human infection was documented only in an American Hantavirus species (Andes virus).4 To date, nine Hantavirus species are implicated in causing HFRS in Europe and Asia (mortality rates ranging between 0-12%) as shown on Table 1, and twenty species are implicated in causing HPS (mortality rates 30-50%).1 However, studies of wildlife in endemic regions suggest that viral spillover occurs in additional animal hosts, although it remains unclear whether these can transmit Hantavirus infection to humans.5,6
Virus |
Geographic Distribution |
Rodent Carrier |
Dobrava/Belgrade |
Balkans |
Apodemus flavicollis |
Tula |
Europe (Czech Republic, Switzerland, Germany), Russia |
Microtus arvalis |
*Puumala |
Europe (Norway, Finland, Sweden, Belgium, Germany, |
Myodes glareolus |
Saaremaa |
Europe (Germany, Slovakia, Balkans), Russia |
Apodeus agrarius |
Amur/Soochong |
Far East, Russia |
Apodemus peninsulae |
Hantaan |
Russia, China, South Korea |
Apodeus agrarius |
Luxi |
China |
Eothenomys miletus |
Seoul |
South Korea |
Rattus |
Sochi |
Russia |
|
Almost 30.000 cases in Eurasia and several hundred cases in the Americas are reported annually.9 Within the European region, according to Eurosurveillance data, during the period 2008-2015, 27.155 cases of Hantavirus infections were reported, with the majority of patients being between 25-65 years old.10 Their spatial distribution is shown on Figure 1.
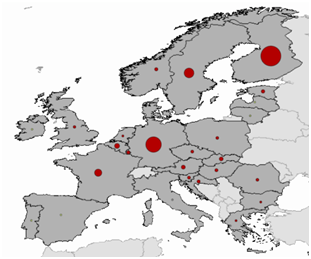
Figure 1 Spatial distribution of Hantavirus infections throughout European countries for the period 2008-201510
Hantavirus infection pathophysiology
Hantaviruses are non-cytopathic, enveloped viruses, members of Bunyaviridae family, with a single-stranded RNA genome (Small “S”, Medium “M” and Large “L” RNA segments), two surface glycoproteins (Gc and Gn) and a Nucleocapsid (N) protein. Viral cell entry is mediated through Hantavirus surface glycoproteins after interaction with host cell membrane proteins.3 Specifically, infective virions attach and enter vascular endothelial cells after interacting with cell surface αvβ3-integrins.11 The pathogenetic mechanism of the underlying vascular permeability increase, during a Hantavirus infection, is common between HFRS and HPS. The endothelial impairment is the main cause of haemorrhagic and renal manifestations in HFRS and in pulmonary manifestations in HPS, as it is described in detail on subsection 5.2. It involves an 18- to 23-fold increase of capillary and vascular permeability through direct or immune-mediated pathways.12 Different co-receptors mediating Hantavirus attachment and entry into the endothelial cells may define some of the differences in clinical manifestations between HFRS and HPS. According to a study, co-factor glycoprotein Decay-accelerating factor (DAF)/CD55, found on the endothelial apical surface, mediates the viral entry of Hantavirus causing specifically HFRS. Furthermore, coating of target human cells by Hantavirus virions, blocks β3-integrins from regulating hemostatic mechanism and vascular permeability, by impairing cell response to VEGF.13 The same effect is manifested also from Vascular Endothelial (VE)-cadherin internalization, which results from Hantavirus cell entry.14 Furthermore the presence of β3-integrins on platelet cell surface and its interaction with virions causes defective platelet activation and thrombocytopenia.13
Cytokine release syndrome (with increased release of interferon-γ, IL-1, IL-2, IL-4, IL-6, TNFα by activated macrophages and dendritic cells) and immune system hyper activation increases endothelial cell permeability. Furthermore, expression of viral antigens on the endothelial cell membrane, up regulates CD8+ Cytotoxic T-lymphocytes, enhancing vascular permeability and contributing to Hantavirus syndromes manifestations. Moreover, immune-complex depositions and complement activation are implicated for kidney involvement.14,15
Epidemiology, clinical features and disease severity of hantavirus infection
Hantavirus infections in humans present as one of two clinical syndromes, with infection incidence being three-fold in males than females. HFRS is predominant in European and Asian region, whereas HPS is the main syndrome caused by Hantavirus in US. HFRS presents usually as a 5-stage disease (Figure 2), following a 2-4 week incubation period.16 During the Prodromal Stage (3-5 days) the patient is febrile with concomitant constitutional flu-like symptoms (headache, malaise, myalgia, anorexia, eye pain), whereas haemorrhagic manifestations (subconjunctival haemorrhage, petechiae and mucous membrane injection) may coexist.2 In addition, cases of Pumala virus infection may present visual disturbances and photophobia (due to lens thickening) as well as neurological complications that may involve epileptic seizures, hemiparesis, structural central nervous system (CNS) lesions, encephalitis, Guillain-Barre syndrome and cranial nerve palsies.9,17 According to literature pathogenesis of CNS involvement is not well understood, and it is considered a part of an autoimmune process, due to molecular mimicry of viral antigens and neural host antigens. An example is Acute Disseminated Encephalomyelitis (ADEM) caused by Pumala virus.9,17
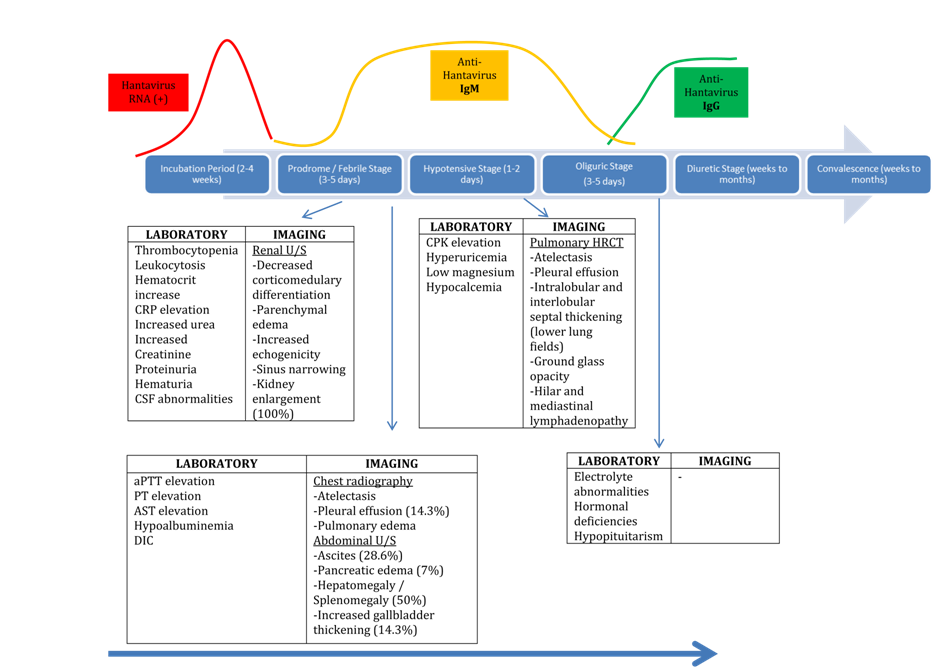
Figure 2 Natural history of Hantavirus infection with relevant serological, PCR, laboratory and imaging findings in each stage9
Patients during the subsequent Hypotensive stage (1-2 days) present clinical signs of hypovolemic shock (up to 21-28%), with concomitant gastrointestinal symptoms (abdominal pain, nausea, vomiting) or signs of acute abdominal pain.16 The wide ranges of exhibited manifestations are not specific during this stage, with wide differential diagnosis, restraining the further diagnosis process. Renal failure of varying severity is the main characteristic of the subsequent Oliguric phase (3-5 days). A proportion of 13-47% of these patients may require haemodialysis. During this phase ECG abnormalities are common with 38% of patients in some series presenting rhythm and conduction abnormalities.18 The Polyuric and Convalescence stages follow (weeks to months), with patient clinical improvement, and synchronous electrolyte abnormalities due to polyuria (reaching 3-6 liters of urine output daily).16 In cases of Nephropathica Epidemica (NE) caused by Puumala virus, nephrotic syndrome or glomerulonephritis may be present.19,20
Each patient may manifest the disease with any combination of clinical or laboratory features and variable severity during its natural history, as shown in Figure 2, making diagnosis of these infections a challenge. Dobrava (DOBV), Puumala (PUUV) and Saaremaa (SAAV) viruses, all cause HFRS. However these infections differ considerably in severity. Common characteristics comprise of acute onset, fever, headache, abdominal pain, back pain, temporary renal failure (initially oliguria, proteinuria, elevated serum creatinine, and polyuria) and thrombocytopenia. The intensity of haemorrhagic manifestations such as hematuria, petechiae, internal haemorrhages and requirement of dialysis treatment, hypotension and fatality incidences are much greater in HFRS caused by DOBV, as compared by NE caused by PUUV or SAAV. Mortality rates in PUUV-HFRS are very low (~0.1%) in comparison with 12% in DOBV infections.2,3 Patients infected by DOBV present usually more severe symptoms with haemorrhagic manifestations being most dominant (26-59%). In addition, severe thrombocytopenia, shock (21-28%), DIC, and renal failure requiring hemodialysis (30-47%), and ascitic/pleural effusions are usually caused by DOBV infections. Furthermore impairment of gastrointestinal tract and electrocardiography findings are often found. Life-threatening neurological manifestations have been also described in some DOBV cases.2,3,9
Prediction of disease severity and risk stratification for presenting complications or high mortality is very important, since a number of patients may be treated on an outpatient basis, especially in endemic areas (Russia, Northern Europe, and China). Several biomarkers and imaging findings during patient evaluation have been found to predict increased infection severity, and increased likelihood of clinical and laboratory such as: elevation of IL-10, TNFα, SC5-9 complement protein, serum Fibronectin, urinary Cystatin-C, viral load and decrease of IL-12 to IL-10 Ratio, C3 complement proteins as well as gallbladder wall thickness greater than 4mm as previously review by Sargianou et al.9
On the other hand HPS that is rarely found in European region is generally a more severe disease than HFRS, with higher mortality rates (30-50%). Its clinical course presents in the second stage with cardiopulmonary manifestations, characterized by hypoxemia, cardiogenic shock, and respiratory failure due to pulmonary infiltrations or pulmonary edema.21
Diagnosis, treatment and prevention of disease
Diagnosis of Hantavirus infections is based on obtaining a detailed past medical history, and thorough physical examination along with compatible laboratory tests. Risk factors for Hantavirus infection involve participation in outdoor activities (rural or forest ones), presence of rodents in the patients domestic environment or inhalation of potentially contaminated dust. In addition contact of infected individuals by Andes virus may predispose for human-to-human infection.8 Presence of risk factors for the infection along with clinical findings such as high fever, with constitutional symptoms and bleeding diathesis (petechiae, subconjunctival haemorrhages) and pathological laboratory findings of thrombocytopenia, leukocytosis, acute renal failure, proteinuria and haematuria should alert the physician for possible HFRS or HPS (Figure 2).
Since early signs of infection are non-specific, laboratory diagnosis of suspected cases is critical in establishing the diagnosis of Hantavirus infection. Serological tests, with detection of specific Anti-hantavirus IgM and IgG antibodies in the serum at the onset of symptoms, using ELISA or IgM Capture ELISA have a major role.21 The latter has been shown to have higher sensitivity and specificity for the infection.22 Indirect Immunofluorscence Assays (IFAs) despite their lower specificity are still used regularly.21 In addition commercially available kits with Rapid 5-minute Immunochromatographic IgM tests have made diagnosis user friendly and rapid.23
Hantavirus infection can be confirmed by detecting viraemia with conventional and quantitative Real-Time Polymerase Chain Reaction (RT-PCR), with higher viraemia presenting in severe infections.24 Viraemia is also present before the formation of specific anti-hantavirus antibodies.24 Other diagnostic tools (Microarray, Next Generation Sequencing, Cell culture isolation) are used mainly in virus discovery and further virological and functional studies, being however too expensive and complex for routing use in clinical practice.18
The management of Hantavirus infections remains at present primarily supportive, since there is no specific approved therapy available. Patients need careful monitoring, intravenous hydration with crystalloid solutions or colloid plasma expanders and correction of electrolyte disturbances. Patients with hypotension not responding to intravenous hydration may need vasopressors, and those with severe fluid overload, pulmonary oedema, metabolic acidosis or severe hyperkalemia are candidates for haemodialysis treatment. In addition in severe thrombocytopenia with bleeding manifestations or DIC, blood, platelet or fresh frozen plasma transfusions may be used. Despite the lack of evidence corticosteroids are sometimes used.21
Severe cases of HFRS and HPS should be admitted in ICUs for appropriate monitoring.25 Supplemental oxygen or mechanical ventilation when indicated, as well as ECMO should be considered.26,27 Ribavirin, a nucleoside analogue, has been shown to inhibit the expression of viral cRNA, mRNA and protein of Hantaan virus through the formation of ribavirin triphosphate intracellularly.28 Ribavirin diffuses into the host cell’s cytoplasm, through cellular membrane, and is phosphorylated by cellular kinases. Its mechanism of action includes mutagenesis to the viral genome, inhibition of the viral mRNA capping and partial suppression of cytokines action.28 Furthermore this drug also pairs with Uridine and Cytidine inhibiting the function of viral RNA-dependent RNA polymerase, resulting delay of the initiation and elongations phases of mRNA transcription.29 It has been shown that administration of Ribavirin intravenously, during the first 5 days after symptom onset of HFRS, reduced mortality rate significantly and reduced the occurrence of severe renal failure.30 However no clinical benefit has been shown after administration of intravenous Ribavirin in HPS patients.31
Prevention of Hantavirus infections may include decreasing occupational or other exposure to aerosolized dust that could be contaminated with viral antigens. Taking necessary measures for avoiding any risk factors, when possible, and prevention of rodent inhabitation near homes and work areas should be sought. An inactivated vaccine has been developed in Korea, with uncertain protective efficacy.32 Further vaccine development is on the way, based on approaches that use recombinant virus proteins, recombinant viruses or DNA vaccines.32
Hantavirus infections are considered emerging zoonotic infectious diseases in the European region and our understanding and recognition of the disease has improved significantly in recent years. Since these infections remain a public health threat, further research on Hantavirus pathogenesis, diagnostic tools and new reservoir host discovery remain a priority. Moreover the apparent effect of Ribavirin on Hantaviruses, has turned drug research to new nucleoside analogues found to be effective in vitro and in animal studies. For example 1-β-d-ribofuranosyl-3-ethynyl-triazole (ETAR).1,2,4 has showed significant antiviral activity against hantavirus when administered in doses 12.5-25mg/kg intraperitoneally.33 On the other hand Arbidol, an immunomodulatory drug, was found to be effective in doses of approximately 80mg/kg/day orally.34 Other approaches such as passive immunization, immunomodulation with recombinant IL-2 or recombinant IFN-a2b, in animal and human studies, have shown also promising results.35 Thus further research and development of new immunomodulatory and antiviral drugs as treatments and other preventive strategies is considered mandatory.
None.
None.
None.

©2017 Ioannou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.