Journal of
eISSN: 2373-437X


Research Article Volume 9 Issue 5
1Forest Research Institute, Hellenic Agricultural Organization– DEMETER, Greece
2Laboratory of Ecology, Department of Biological Applications and Technology, University of Ioannina, Greece
3Forest Service of Ioannina, Greece
Correspondence: Stephanos Diamandis, Forest Research Institute, Hellenic Agricultural Organization – DEMETER, 57006 Vassilika, Greece, Tel +306932584607
Received: August 24, 2021 | Published: September 20, 2021
Citation: Diamandis S, Topalidou E, Avtzis D, et al. Fungal diversity in sacred groves vs. managed forests in Epirus, NW Greece. J Microbial Exp. 2021;9(5):142-154. DOI: 10.15406/jmen.2021.09.00335
Fungal diversity and yield based on sporophore production was assessed in sacred groves of Epirus NW Greece and was compared to nearby forests. Sacred groves are woodland surrounding old chapels or monasteries which have been left undisturbed and hold old-growth trees. In eight sites, plots were set in sacred groves and also in managed coppice forests (as controls). Sampling was conducted in 2013, 2014, 2015. The 208 fungal taxa recorded were classified as ectomycorrhizal (ECM), saprotrophic and xylotrophic. Sacred groves were found to hold greater species richness and higher yield but when fungal groups were compared individually, yield of ECM fungi was higher in control sites. On the basis of the current research it was found that: 1) Sacred groves hold more fungal diversity of xylotrophic and saprotrophic fungi. 2) ECM fungi are more productive in younger, managed forests. 3) Even with clearcut logging on a 30-yr rotation, fungal diversity remains high. These results should be considered when managing old-growth woods for fungal conservation.
Keywords: sacred groves, state-managed coppice forest, fungal diversity, species richness, sporophore yield, conservation
Old-growth forests are rare in Europe, particularly in the Mediterranean basin, because of a long history of human exploitation.¹ In Epirus, NW Greece, ruined chapels, remote churches and abandoned monasteries which are scattered throughout the countryside are commonly surrounded by old trees, groves or woodland.² Such “Sacred Natural Sites” have survived through time due to the fact that local people paid religious respect to these areas and avoided disturbing them for their personal use with activities such as cutting timber, harvesting firewood or grazing their animals. These ecosystems were established and flourished during the Ottoman occupation (1479–1912) when communities, along with Church authorities, imposed controlled management in sacred forests through social taboos. Management, or in this case non-management, of these sacred groves created patches of undisturbed, old-growth woodland.
Sacred Natural Sites are considered crucial for conservation of biodiversity.3-5 However, in most cases, the assessment of sacred sites for biodiversity potential was based solely on specific groups of organisms and mainly flora.6-8 Although fungi are key biological components of forest ecosystems they remain a relatively under-studied and under-protected kingdom.9,10 Fungal diversity on its own in sacred groves has seldom been investigated. To compound this neglect, fungi, “The orphans of Rio”, have been excluded from all protection schemes, including the NATURΑ 2000 areas in EU which is considered as one of the most succesfull networks worldwide, ignoring in this way an entire kingdom of organisms crusial to nature. (see http://www.fungal-conservation.org/blogs/orphans-of-rio.pdf). Furthermore, there is no specific law in Greece for the protection of fungi or any species specific regulations pertaining to picking and marketing of edible fungal species.11
In the remote region of Epirus (Figure 1) there are numerous sacred groves of various sizes. They form habitats dominated by mature trees that are unique within landscapes which have been intensively used throughout history.12,13 Sacred forests can either belong to local communities or to the State, however, their management is traditionally defined by the Church. On the other hand, State-owned young evergreen or mixed broadleaved forests in the region are managed by the Forest Service. They are mostly coppice forests clearcut every 30 yrs but also include pastureland mixed with recently abandoned agricultural land.
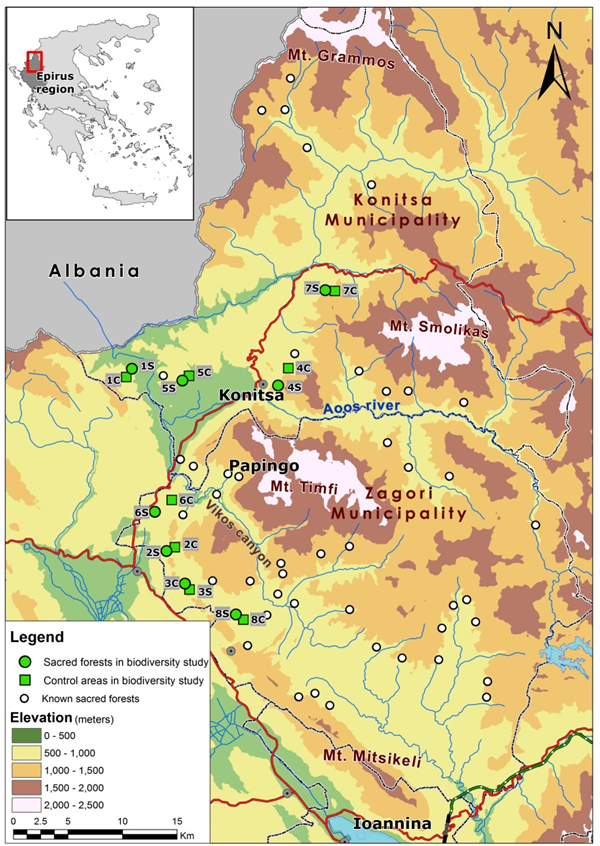
Figure 1 At least 80 SNS have been identified in the broad area of Zagori and Konitsa (white cyrcles). For the current study, we measured fungal biodiversity in eight of these sacred groves (green circles) and in the eight matching control sites (green squares). (From Avtzis et al. 2019).
The objective of the present work was to assess the fungal diversity of above ground macromycetes encountered in sacred groves (both in species richness and yield) based on sporophore production and compare the results with the fungi which occur in nearby State-managed forests with similar topographic and vegetative features. The null hypothesis that sacred, undisturbed groves are richer in fungal diversity and yield than managed forests was tested.
Study areas
The present investigation was conducted in the mountains of the municipalities of Zagori and Konitsa in the region of Epirus (NW Greece) near the Albanian border (Figure 1). Sacred groves in this area have been indentified in the majority of the 80 villages of the area as a result of long-term ethnographic research. Research sites were selected from the extended network of sacred groves occurring there based on historical management and ecological criteria.13
Eight sacred groves (marked as 1S-8S) which ranged from 0.15 to 64.26 ha (Table 1) were selected for the current study representing three different floral communities: conifers, broadleaved evergreens, and deciduous broadleaved forests. In each grove, one rectangular plot, 10*20m in size was selected. For each sacred grove, a matching control site (marked as 1C-8C) equal in size was selected in nearby managed forest in the closest possible proximity featuring identical topographic position and floral composition to allow direct comparison between sacred groves and managed forest. In most cases the distance between sacred and control plots was less than 2 km. Sites 4 and 7 (Figure 1) had steeper slopes and were dominated by conifers. The other six sites were located in lowlands or southern-aspect slopes and were dominated by broadleaved trees. The striking difference between sacred and control sites was that the former were undisturbed with large, aged trees situated well apart with gaps in the canopy and a good amount of coarse woody debris,14 while in the later, the trees were young, aged 15-20 yrs, dense and fast growing because of regular management as coppice forests, or due to recent abandonement of grazing with mostly thin woody debris.
|
Sites |
Ectomycorrhizal (ECM) |
Saprotrophic |
Xylotrophic |
|||
|
Plots |
S |
C |
S |
C |
S |
C |
|
1.Aidonochori |
5 |
14* |
18 |
13 |
24 |
18 |
|
2.Elaphotopos |
10 |
10 |
8 |
8 |
16 |
11 |
|
3.Kato Pedina |
7 |
11 |
14 |
10 |
18* |
11 |
|
4.Konitsa |
13 |
8 |
7 |
8 |
6 |
8 |
|
5.Mazi |
7 |
9 |
5 |
3 |
5 |
5 |
|
6.Mesovouni |
9* |
0 |
14 |
11 |
9 |
17* |
|
7.Molista |
8 |
12 |
26* |
18 |
12* |
5 |
|
8.Vitsa |
13* |
5 |
12 |
7 |
11* |
5 |
Table 1 Incidence of each ecotrophic group within and across the 8 pairs of sacred and control plots (S-sacred, C-control). Asterisks (*) denote that significant differences (p<0.05 or p<0.001, ANOVA contrasts) within each site were detected between sacred and matching control plots and were tested separately for each ecotrophic group
The tree vegetation types encountered in the 8 sampling sites (referred to with the name of the nearest village) were:
|
Name of site |
Vegetation type |
|
1. Aidonochori |
Ostrya carpinifolia, Quercus frainetto, Acer monspennsulanum, Acer obtusatum, (mixed broadleaved, oak dominated forest). |
|
2. Elaphotopos |
Carpinus orientalis, Quercus coccifera, Fraxinus ornus, Ruscus aculeatus, (evergreen broadleaved dominated forest). |
|
3. Kato Pedina |
Acer monspenssulanum, Quercus coccifera, Carpinus orientalis, Hedera helix, (evergreen broadleaved dominated forest). |
|
4. Konitsa |
Pinus nigra, Quercus coccifera, Juniperus oxycedrus, Corylus avellana (evergreen broadleaved dominated forest). |
|
5. Mazi |
Carpinus orientalis, Quercus coccifera, Q. cerris, Q. pubescens, Q. frainetto, Juniperus oxycedrus, (mixed broadleaved, oak dominated forest). |
|
6. Mesovouni |
Carpinus orientalis, Quercus coccifera, Acer monspenssulanum, Ruscus aculeatus, (evergreen broadleaved dominated forest). |
|
7. Molista |
Pinus nigra, Abies borisii regis, Corylus avellana, Acer obtusatum, Carpinus orientalis, (coniferous dominated forest). |
|
8. Vitsa |
Quercus frainetto, Carpinus orientalis, Quercus coccifera, Juniperus oxycedrus, (mixed broadleaved, oak dominated forest). |
Assessment of incidence and sporophore yield was accomplished by carefully examining each 200m² plot. The mushroom data collection was focused on epigeous macrofungi which could be easily observed and assessed. The sporophores found were first identified to species in situ according to their morphological features and then were counted. In case of doubtful identification, sporophores were counted by species and then specimens were taken in portable coolers to the Forest Research Institute for laboratory examination and identification. All collected specimens were appropriately treated before being deposited in the Fungal Collection of the Forest Research Institute.
Assessment and sampling within these eight sites was conducted in two weekly visits with an interval of one week during three consecutive fall periods of the years 2013, 2014 and 2015 when the typical peak in mushroom fruiting occurred. Sacred groves and control sites were compared in terms of species richness and yield of sporophores per site. All individual sporophores assessed were identified to species level (or taxa when necessary) and classified into four ecotrophic groups according to their trophic status: ectomycorrhizal (ECM), saprotrophic, xylotrophic and parasitic (Table 1). Resupinate species were considered as colonies and not as individual sporophores (Table 5). Specimens which were identified only to genus level are marked as sp. Species taxonomy and nomenclature followed that of Index Fungorum.
Statistical analyses
Statistical analysis to test the effect of forest management (sacred vs. control) on species richness and on sporophore production was carried out at site and plot level. Comparisons of incidence among and within the eight sites were evaluated by using linear mixed models with the method of Restricted Maximum Likelihood (REML). This particular method is commonly utilized as the best way to estimate variance parameters since it is unbiased and better suited to unbalanced survey data.15-18 Sites and plots were specified as fixed factors whereas the ecotrophic groups were considered as random factors and their effects were calculated through the covariate model. The response variables were (a) species incidence for each taxon and (b) yield of sporophores for each taxon. Where REML estimate showed significant effects of forest management in sacred and control plots or significant interactions with the examined diversity levels, ANOVA contrasts (General ANOVA) were used in order to test whether differences among groups were statistically significant.19,18 A simple paired t-test was also applied for the comparison of the totals (among the different ecotrophic groups) for the different forest management types (sacred or control). The facilities in Genstat v12 (VSN International Ltd, Hemel Hempsted-Hertfordshire, UK) were used for the statistical analysis.
In total 2,326 sporophores belonging to 208 species were observed and identified within the eight pairs of sacred and control plots. Lists with cumulative data over a total sampling area of 3,200 m² and the 3 sampling years appear in Tables 3, 4, 5 and 6 in the Appendix. In the group of parasitic fungi (Table 6), assessment of the abundance of the 15 species that were observed was not possible or assessable, given that the most common parasitic fungi were mildew (Erisyphe alphidoides) on oak leaves, tar spot disease (Rhytisma acerinum) on maple, Coccomyces delta and C. dentatus on Kermes oak leaves. A few other micromycetes such as Cyclaneusma minus, Gymnosporangium sabinae, Ramularia endophylla are mentioned in Table 6, however, these were not evaluated. The cumulative data for the three remaining groups are presented in Tables 1 and 2 and Figures 2 and 3.
|
Sites |
Ectomycorrhizal (ECM) |
Saprotrophic |
Xylotrophic |
Total No of sporophores |
||||
|
Plots |
S |
C |
S |
C |
S |
C |
S |
C |
|
1.Aidonochori |
10 |
52* |
60 |
40 |
140* |
42 |
210 |
134 |
|
2.Elaphotopos |
26 |
27 |
21 |
28 |
80 |
50 |
127 |
105 |
|
3.Kato Pedina |
10 |
23 |
111 |
74 |
153* |
58 |
274 |
155 |
|
4.Konitsa |
39 |
34 |
32 |
97 |
38 |
55 |
109 |
186 |
|
5.Mazi |
13 |
35* |
36* |
12 |
51 |
32 |
100 |
79 |
|
6.Mesovouni |
29* |
0 |
79* |
26 |
75 |
63 |
183 |
89 |
|
7.Molista |
30 |
44 |
147* |
62 |
50* |
18 |
227 |
124 |
|
8.Vitsa |
33 |
30 |
36 |
20 |
92* |
13 |
161 |
63 |
|
Totals |
190 |
245 |
522* |
359* |
679* |
331 |
1.391* |
935 |
Table 2 Number of sporophores of each ecotrophic group within and across the 8 pairs of sacred and control plots (S-sacred, C-control). Asterisks (*) denote that significant differences (p<0.05 or p<0.001, ANOVA contrasts) within each site were detected between sacred and matching control plots and were tested separately for each ecotrophic group
|
Species |
Number of sporophores |
|||||||||||||||
|
Sites |
Aido |
Elap |
KatP |
Kon |
Mazi |
Mes |
Mol |
Vitsa |
||||||||
|
Plots (numbers 1 to 8 refer to sites, S-sacred, C-control) |
S1 |
C1 |
S2 |
C2 |
S3 |
C3 |
S4 |
C4 |
S5 |
C5 |
S6 |
C6 |
S7 |
C7 |
S8 |
C8 |
|
1.Aureoboletus gentilis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2. Amanita battarrae |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
|
3. Amanita caesarea |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
4. Amanita lividopallescens |
0 |
0 |
0 |
0 |
3 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
4 |
|
5. Cantharellus cibarius |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
7 |
|
6. Chroogomphus rutilus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
|
7. Cortinarius bivelus* |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
8. Cortinarius infractus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
9. Cortinarius sp. |
0 |
0 |
0 |
0 |
1 |
2 |
0 |
0 |
1 |
4 |
0 |
0 |
0 |
1 |
1 |
0 |
|
10. Cortinarius trivialis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
2 |
0 |
|
11. Gyroporus castaneus |
0 |
0 |
4 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
12. Hygrophorus eburneus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
13. Hygrophorus olivaceoalbus |
2 |
7 |
0 |
0 |
0 |
0 |
3 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
14. Hygrophorus pudorinus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
15. Hygrophorus russula |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
16. Inocybe albomarginata |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
17. Inocybe asterospora |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
|
18. Inocybe geophylla |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
|
19. Inocybe griseolilacina |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
20. Inocybe hirtella |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
|
21. Inocybe personata |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
22. Inocybe sindonia |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
23. Inocybe sp. |
0 |
0 |
1 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
24. Inocybe tenebrosa |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
25. Inosperma maculatum |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
26. Lactarius acerrimus |
0 |
0 |
0 |
0 |
1 |
2 |
0 |
0 |
2 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
|
27. Lactarius deliciosus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
1 |
0 |
|
28. Lactarius piperatus |
0 |
0 |
4 |
7 |
0 |
2 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
|
29. Lactarius sanguifluus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
|
30. Lactarius subdulcis |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
31. Pseudosperma rimosum |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
|
32. Ramaria largentii |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
|
33. Ramaria rubella |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
|
34. Russula alutacea |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
35. Russula atropurpurea |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
36. Russula aurea |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
1 |
5 |
|
37. Russula carpini |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
4 |
|
38. Russula curtipes |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
39. Russula cyanoxantha |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
40. Russula delica |
0 |
3 |
5 |
4 |
0 |
2 |
2 |
0 |
0 |
4 |
0 |
0 |
0 |
2 |
1 |
10 |
|
41. Russula densifolia |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
42. Russula grata |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
43. Russula heterophylla |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
|
44. Russula insignis |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
45. Russula lilacea |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
|
46. Russula maculata |
0 |
0 |
2 |
1 |
0 |
0 |
2 |
0 |
1 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
|
47. Russula ochroleuca |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
48. Russula pallidospora |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
49. Russula pectinatoides |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
50. Russula romelli |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
|
51. Russula rosea |
0 |
9 |
0 |
2 |
2 |
2 |
0 |
0 |
5 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
|
52. Russula sp. |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
7 |
0 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
|
53. Russula torulosa |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
|
54. Russula vesca |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
|
55. Russula veternosa |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
0 |
0 |
0 |
|
56. Russula unicolor |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
57. Suillus granulatus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
|
58. Suillus luteus |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
0 |
|
59. Suillus variegates |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
60. Tricholoma acerbum |
0 |
8 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
61. Tricholoma caligatum |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
|
62. Tricholoma gausapatum |
0 |
0 |
0 |
4 |
0 |
0 |
5 |
3 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
|
63. Tricholoma lascivum* |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
|
64. Tricholoma pardinum |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
8 |
0 |
0 |
0 |
|
65. Tricholoma scalpturatum |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
9 |
0 |
|
66. Tricholoma terreum |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
0 |
0 |
|
67. Tricholoma ustale |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
|
68. Xerocomellus chrysenteron |
1 |
0 |
5 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Table 3 List of ECM fungi recorded in all eight sites in the sacred (S) and their corresponding control (C) plots
|
Species |
Number of sporophores |
|||||||||||||||
|
Sites |
Aido |
Elap |
KatP |
Kon |
Mazi |
Mes |
Mol |
Vitsa |
||||||||
|
Plots (numbers 1 to 8 refer to sites) |
S1 |
C1 |
S2 |
C2 |
S3 |
C3 |
S4 |
C4 |
S5 |
C5 |
S6 |
C6 |
S7 |
C7 |
S8 |
C8 |
|
1.Armillaria mellea |
4 |
3 |
0 |
0 |
0 |
8 |
0 |
0 |
15 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
|
2. Athelia neuhoffii |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
3. Auriscalpium vulgare |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
9 |
0 |
0 |
0 |
0 |
8 |
1 |
0 |
0 |
|
4. Bolbitius titubans var. titubans |
0 |
2 |
4 |
0 |
8 |
0 |
0 |
0 |
0 |
0 |
4 |
1 |
0 |
0 |
0 |
0 |
|
5. Calocybe carnea |
1 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
1 |
0 |
0 |
|
6. Clavariadelphus truncates |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
1 |
0 |
0 |
|
7. Collybia sp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
|
8. Conocybe rickenii |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
9. Coprinellus impatiens |
0 |
0 |
3 |
0 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
|
10. Coprinellus truncorum |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
11. Coprinellus xanthothrix |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
12. Coprinopsis picacea |
3 |
5 |
0 |
0 |
5 |
0 |
0 |
0 |
5 |
2 |
0 |
1 |
0 |
0 |
0 |
2 |
|
13. Cystoderma amiantinum |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
2 |
0 |
0 |
|
14. Cystodermella granulosa |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
1 |
0 |
0 |
|
15. Discina ancilis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
|
16. Entoloma chalybaeum var. chalybaeum |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
2 |
0 |
0 |
|
17. Gymnopus aquosus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
3 |
0 |
0 |
|
18. Gymnopus brassicolens |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
34 |
0 |
0 |
0 |
0 |
12 |
0 |
0 |
0 |
|
19. Gymnopus dryophilus |
0 |
8 |
0 |
0 |
7 |
3 |
0 |
12 |
7 |
8 |
1 |
3 |
0 |
0 |
4 |
0 |
|
20. Gymnopus hariolorum |
0 |
5 |
7 |
0 |
7 |
5 |
0 |
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
21. Helvella acetabulum |
2 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
0 |
1 |
0 |
|
22. Helvella atra |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
23. Helvella leucomelaena |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
2 |
0 |
0 |
|
24. Hemimycena lactea |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
4 |
0 |
0 |
0 |
0 |
10 |
9 |
0 |
0 |
|
25. Hyaloscypha hyalina |
1 |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
0 |
0 |
|
26. Hydropus conicus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
|
27. Hydropus marginellus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
|
28. Hygrophorus pratensis var. pratensis |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
29. Lepiota brunneoincarnata |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
30. Lepiota sp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
|
31. Lepiota sublaevigata |
3 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
0 |
0 |
0 |
3 |
|
32. Lycoperdon nigrescens |
0 |
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
33. Lycoperdon perlatum |
11 |
0 |
1 |
0 |
0 |
7 |
2 |
0 |
0 |
0 |
5 |
0 |
27 |
17 |
0 |
0 |
|
34. Marasmius cohaerens |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
9 |
0 |
0 |
0 |
|
35. Marasmius epiphyllus |
5 |
0 |
0 |
0 |
16 |
4 |
0 |
30 |
0 |
0 |
27 |
3 |
0 |
0 |
0 |
0 |
|
36. Marasmius quercophilus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
|
37. Melanoleuca exscissa |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
38. Melanoleuca rasilis * |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
1 |
2 |
0 |
|
39. Mycena acicula |
5 |
0 |
0 |
13 |
18 |
24 |
0 |
0 |
3 |
0 |
7 |
3 |
4 |
1 |
0 |
5 |
|
40. Mycena epipterygia |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
0 |
|
41. Mycena galopus |
0 |
1 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
9 |
0 |
0 |
0 |
0 |
0 |
|
42. Mycena leptocephala |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
4 |
0 |
0 |
|
43. Mycena polyadelpha |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
6 |
0 |
0 |
|
44. Mycena pura |
2 |
0 |
3 |
0 |
7 |
0 |
0 |
1 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
|
45. Mycena rosea |
4 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
1 |
1 |
|
46. Panaeolus papilionaceus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
47. Peziza succosa |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
2 |
0 |
0 |
|
48. Peziza vesiculosa |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
49. Protostropharia semiglobata |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
1 |
0 |
|
50. Psathyrella murcida |
0 |
1 |
0 |
0 |
7 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
|
51. Psartyrella spadiceogrisea |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
2 |
3 |
0 |
0 |
0 |
|
52. Psathyrella sp. |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
|
53. Psathyrella tephrophylla |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
54. Resupinatus applicatus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
55. Rhodocollybia butyracea |
3 |
0 |
0 |
0 |
8 |
0 |
0 |
0 |
0 |
2 |
4 |
0 |
0 |
0 |
1 |
4 |
|
57. Sarcosphaera coronaria |
0 |
0 |
0 |
0 |
0 |
0 |
9 |
0 |
0 |
0 |
0 |
0 |
4 |
1 |
0 |
0 |
|
56. Scleroderma citrinum |
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
58. Scleroderma sp. |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
59. Scleroderma verrucosum |
0 |
2 |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
|
60. Sphaerobolus stellatus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
0 |
|
61. Strobilurus tenacellus |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
62. Stropharia aeruginosa |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
6 |
0 |
5 |
0 |
1 |
0 |
0 |
0 |
|
63. Verpa conica |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
64. Verpa digitaliformis |
0 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Table 4 List of saprotrophic fungi recorded in all eight sites in the sacred (S) and their corresponding control (C) plots
|
Species |
Number of sporophores |
|||||||||||||||
|
Sites |
Aido |
Elap |
KatP |
Kon |
Mazi |
Mes |
Mol |
Vitsa |
||||||||
|
Plots (numbers 1 to 8 refer to sites) |
S1 |
C1 |
S2 |
C2 |
S3 |
C3 |
S4 |
C4 |
S5 |
C5 |
S6 |
C6 |
S7 |
C7 |
S8 |
C8 |
|
1. Auricilaria auricula-judae (colonies) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
|
2. Auricularia mesenterica (colonies) |
0 |
1 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
|
3. Byssomerulius corium (colonies) |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
4. Calocera cornea (colonies) |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
|
5. Cerrena unicolor (colonies) |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
6. Crepidotus variabilis |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
1 |
|
7. Cyanosporus subcaesius |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
8. Dacrymyces stillatus (colonies) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
|
9. Daedalea quercina |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
|
10. Daedaleopsis nitida |
0 |
1 |
16 |
12 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
37 |
0 |
|
11. Daldinia concentrica |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
|
12. Dendrothele alliacea (colonies) |
9 |
0 |
7 |
4 |
7 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
13. Dentipellis fragilis (colonies) |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
14. Desarmillaria tabescens |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
9 |
0 |
0 |
0 |
0 |
0 |
0 |
|
15. Exidia glandulosa (colonies) |
4 |
1 |
3 |
0 |
9 |
0 |
13 |
6 |
13 |
6 |
4 |
0 |
0 |
0 |
9 |
5 |
|
16. Exidia nigricans (colonies) |
0 |
1 |
1 |
0 |
3 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
17. Fomitopsis pinicola |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
|
18. Fomitiporia punctata |
6 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
19. Galerina marginata |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
|
20. Galerina marginata |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
21. Ganoderma adspersum |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
22. Ganoderma applanatum |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
23. Ganoderma lucidum |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
24. Gloeoporus taxicola (colonies) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
25. Gymnopus erythropus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
1 |
0 |
|
26. Gymnopus fusipes |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
27. Gymnopus ocior |
0 |
12 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
28. Hymenopellis radicata |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
|
29. Hypholoma acutum |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
15 |
0 |
1 |
0 |
0 |
|
30. Hypoxylon fragiforme (colonies) |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
31. Hypoxylon rubiginosum (colonies) |
0 |
0 |
14 |
12 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
32. Irpex lacteus (colonies) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
33. Junghuhnia nitida (colonies) |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
34. Kretzschmaria deusta (colonies) |
0 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
35. Kuehneromyces mutabilis |
7 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
36. Lentirus brumalis |
2 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
14 |
2 |
|
37. Mucidula mucida |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
38. Mycena arcangeliana |
0 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
39. Mycena galericulata |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
|
40. Mycena inclinata |
0 |
0 |
2 |
1 |
0 |
0 |
0 |
36 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
41. Mycena meliigena |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
42. Mycena purpureofusca |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
0 |
|
43. Mycena renatii |
2 |
0 |
0 |
0 |
43 |
21 |
0 |
0 |
0 |
0 |
7 |
3 |
0 |
0 |
0 |
0 |
|
44. Mycena tenerrima |
0 |
0 |
3 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
45. Mycoacia fuscoatra (colonies) |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
46. Mycoacia uda (colonies) |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
47. Oudemansiella melanotricha |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
48. Panellus mitis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
49. Panellus stipticus |
21 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
3 |
|
50. Peniophora quercina (colonies) |
0 |
1 |
0 |
0 |
4 |
1 |
0 |
0 |
0 |
3 |
9 |
3 |
0 |
0 |
0 |
0 |
|
51. Phanerochaete laevis (colonies) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
|
52. Phellodon niger |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
0 |
0 |
0 |
0 |
0 |
6 |
12 |
0 |
0 |
|
53. Phloeomana speira |
1 |
0 |
7 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
54. Pluteus cervinus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
55.Pluteus salicinus |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
56. Polyporus tuberaster |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
57. Pseudohydnum gelatinosum |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
16 |
0 |
0 |
0 |
|
58. Resupinatus applicatus (colonies) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
59. Sarcodontia pachyodon |
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
60. Schizophyllum commune |
13 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
61. Steccherinum fimbriatum (colonies) |
10 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
62. Stereum gausapatum (colonies) |
10 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
|
63. Stereum hirsutum (colonies) |
8 |
5 |
11 |
11 |
30 |
1 |
12 |
7 |
24 |
7 |
15 |
7 |
0 |
1 |
10 |
2 |
|
64. Stereum subtomentosum |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
|
65. Strobilurus tenacellus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
66. Szczepkamyces campestris |
0 |
2 |
3 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
67. Tapinella panuoides |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
68. Tarzetta catinus |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
0 |
0 |
0 |
0 |
0 |
|
69. Terana coerulea (colonies) |
0 |
1 |
3 |
0 |
0 |
1 |
0 |
0 |
3 |
0 |
5 |
3 |
0 |
0 |
0 |
0 |
|
70. Trametes hirsuta |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
0 |
0 |
|
71. Trametes ochracea |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
72. Trametes versicolor |
0 |
0 |
0 |
0 |
0 |
11 |
0 |
0 |
0 |
7 |
0 |
1 |
0 |
0 |
0 |
0 |
|
73. Tremella mesenterica |
5 |
2 |
0 |
1 |
6 |
2 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
|
74. Trichaptum abietinum (colonies) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
|
75. Vuilleminia comedens (colonies) |
7 |
1 |
6 |
3 |
34 |
11 |
0 |
0 |
0 |
0 |
22 |
6 |
1 |
0 |
0 |
0 |
|
76. Xylaria hypoxylon |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Table 5 List of xylotrophic fungi recorded in all eight sites in the sacred (S) and their corresponding control (C) plots
|
Species |
Presence |
|||||||||||||||
|
Sites |
Aido |
Elap |
KatP |
Kon |
Mazi |
Mes |
Mol |
Vitsa |
||||||||
|
Plots (numbers 1 to 8 refer to sites) |
S1 |
C1 |
S2 |
C2 |
S3 |
C3 |
S4 |
C4 |
S5 |
C5 |
S6 |
C6 |
S7 |
C7 |
S8 |
C8 |
|
1. Ampelomyces quisqualis* |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Y |
Y |
- |
- |
- |
- |
|
2. Anthostoma acerinum * |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Υ |
Ν |
- |
- |
|
3. Anthostomella sp. |
- |
- |
Y |
N |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
4. Coccomyces delta |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Ν |
Υ |
|
5. Coccomyces dentatus. |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Ν |
Υ |
|
6. Cyclaneusma minus |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Υ |
Υ |
- |
- |
|
7. Durandiella gallica |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Υ |
Ν |
- |
- |
|
8. Erysiphe alphidoides |
- |
- |
- |
- |
- |
- |
- |
- |
N |
Y |
- |
- |
Y |
N |
- |
- |
|
9. Gymnosporangium sabinae |
- |
- |
- |
- |
- |
- |
- |
- |
N |
Y |
- |
- |
Y |
N |
- |
- |
|
10. Lophodermium pinastri |
- |
- |
- |
- |
- |
- |
Y |
N |
- |
- |
- |
- |
Y |
Y |
- |
- |
|
11. Pestalotiopsis neglecta |
Υ |
Ν |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
12. Phomopsis pustulata |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Y |
N |
- |
- |
|
13. Ramularia endophylla * |
- |
- |
- |
- |
- |
- |
- |
- |
Υ |
Ν |
- |
- |
- |
- |
- |
- |
|
14. Stemonitis axifera |
Y |
N |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
15. Trichoderma viride |
Ν |
Υ |
- |
- |
- |
- |
- |
- |
- |
- |
Ν |
Υ |
- |
- |
- |
- |
Table 6 List of uncounted parasitic species recorded in all eight sites in the sacred (S) and corresponding control (C) plots. (abrev. Y-yes, N-no)
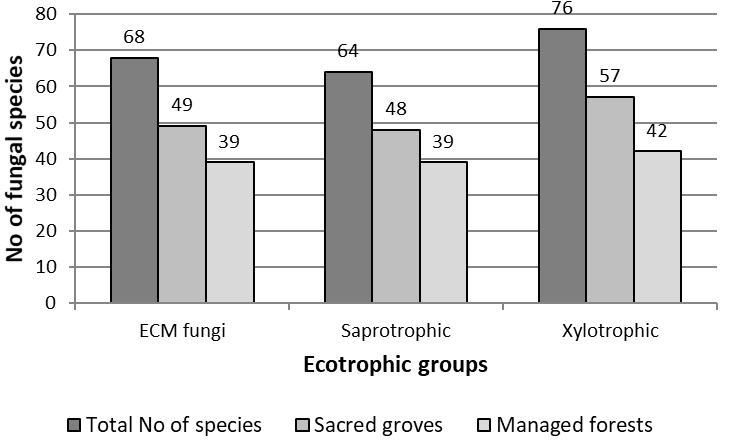
Figure 2 Comparative total incidence per ecotrophic group recorded in the 8 pairs of plots in sacred groves vs. managed forest.
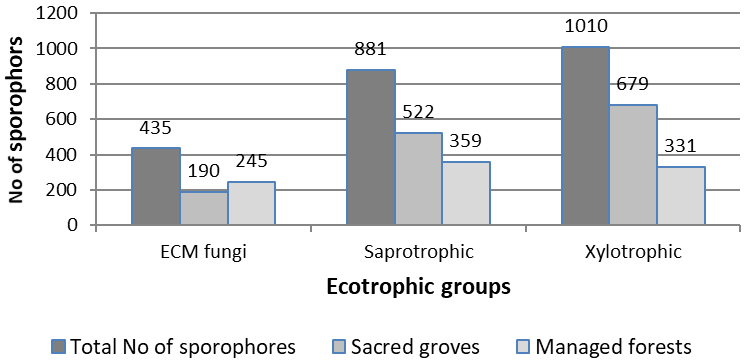
Figure 3 Comparative total yield of per ecotrophic group sporophores recorded in the 8 pairs of plots in sacred groves vs. managed forest.
Out of the 208 species recorded, 72 species were recorded both in sacred and control plots, 87 species were recorded only in sacred plots, while 49 species were recorded only in control plots. The total incidence of fungal species was significantly higher (p<0.001, Wald-test and paired t-test) in sacred plots (Total incidence=154) than in the control plots (Total incidence=120). Differences were significantly affected by the three-way interaction among sites, plots and ecotrophic groups (p<0.001, Wald-test Table 1, Figure 4a). Similarly, the total yield of sporophores was significantly higher (p<0.001, Wald-test and paired t-test) in sacred plots (Total sporophore yield=1,391) than in the control plots (Total sporophore yield=935). Differences were significantly affected again by the three way interaction among sites, plots and ecotrophic groups (p=0.02, Wald-test, Table 2, Figure 5). Variability for both incidence and yield of sporophores was larger in sacred plots (Figures 5a, 5b).
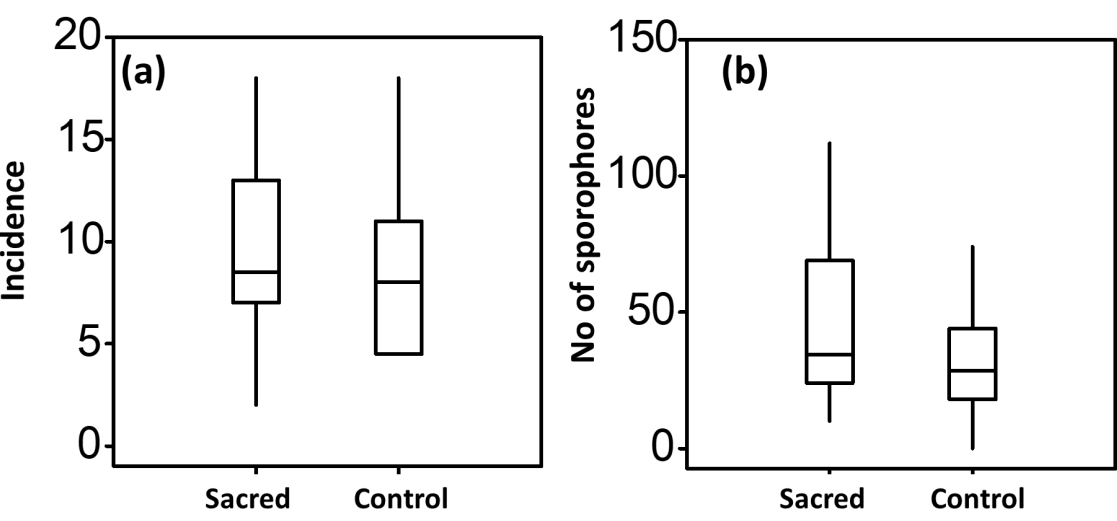
Figure 4 Range, medians and population variability (quartiles) for the (a) incidence of fungal species (mean for the sacred plots was Meansacred=9.8±1.1 and mean for the control plots was Meancontrol=8.1±0.8) and for the (b) number of sporophores (mean for the sacred plots was (Meansacred=48.3±7.4 and mean for the control plots Meancontrol=32.7±4.7).
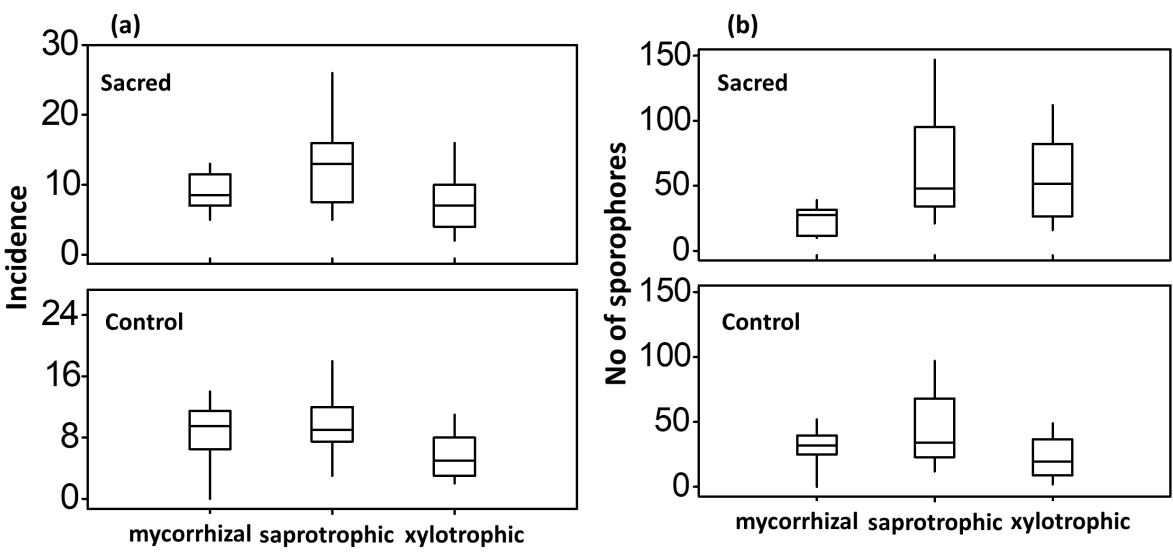
Figure 5 (a) Range, medians and population variability (quartiles) of the three ecotrophic groups recorded in sacred and control plots. Mean incidence for sacred plots was 9.00±1.0, 13.00±2.4 and 7.5±1.6 for ECM, saprotrophic, and xylotrophic fungi, respectively. Mean incidence for the control plots was 8.62±1.6, 9.7±1.6 and 5.9±1.07 for ECM, saprotrophic, xylotrophic fungi, respectively. (b) Range, medians and population variability (quartiles) of the three ecotrophic groups recorded in sacred and control plots. Mean number of sporophores recorded in sacred plots was 23.7±4.0, 65.2±15.7 and 56.0±12.1 for ECM, saprotrophic, xylotrophic fungi, respectively while the mean number of sporophores recorded in the corresponding control plots was 30.6±5.6, 44.9±10.5 and 22.6±6.1.
Incidence among the different ecotrophic groups varied according to the plot classification (sacred or control) as mentioned above, but considerable variation in their incidence was attributed to the three way interaction among sites, plots and ecotrophic groups (p≤0.001, Wald test, Figure 5, Table 1). Similarly, yield of sporophores in each ecotrophic group varied significantly between sacred and control plots (p≤0.001, Wald test, Figure 7) and those differences were again significantly affected by the three way interaction among sites, plots and ecotrophic groups (p=0.02, Wald test, Table 2).
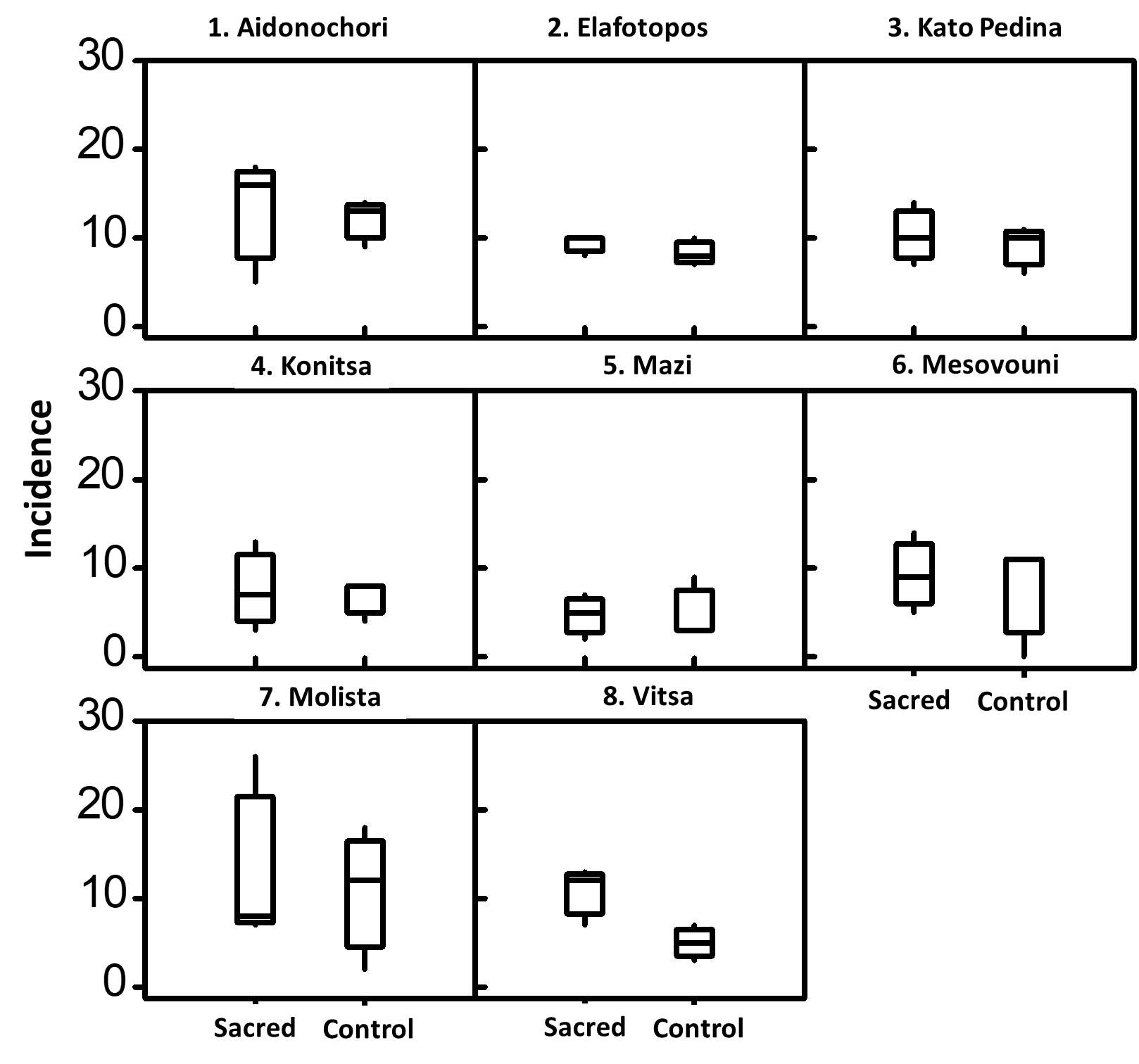
Figure 6 Distribution of the incidence (number of fungal species) across the examined sites. Boxplots show the range, medians and fungal variability (quartiles) between control and sacred plots within each examined site.
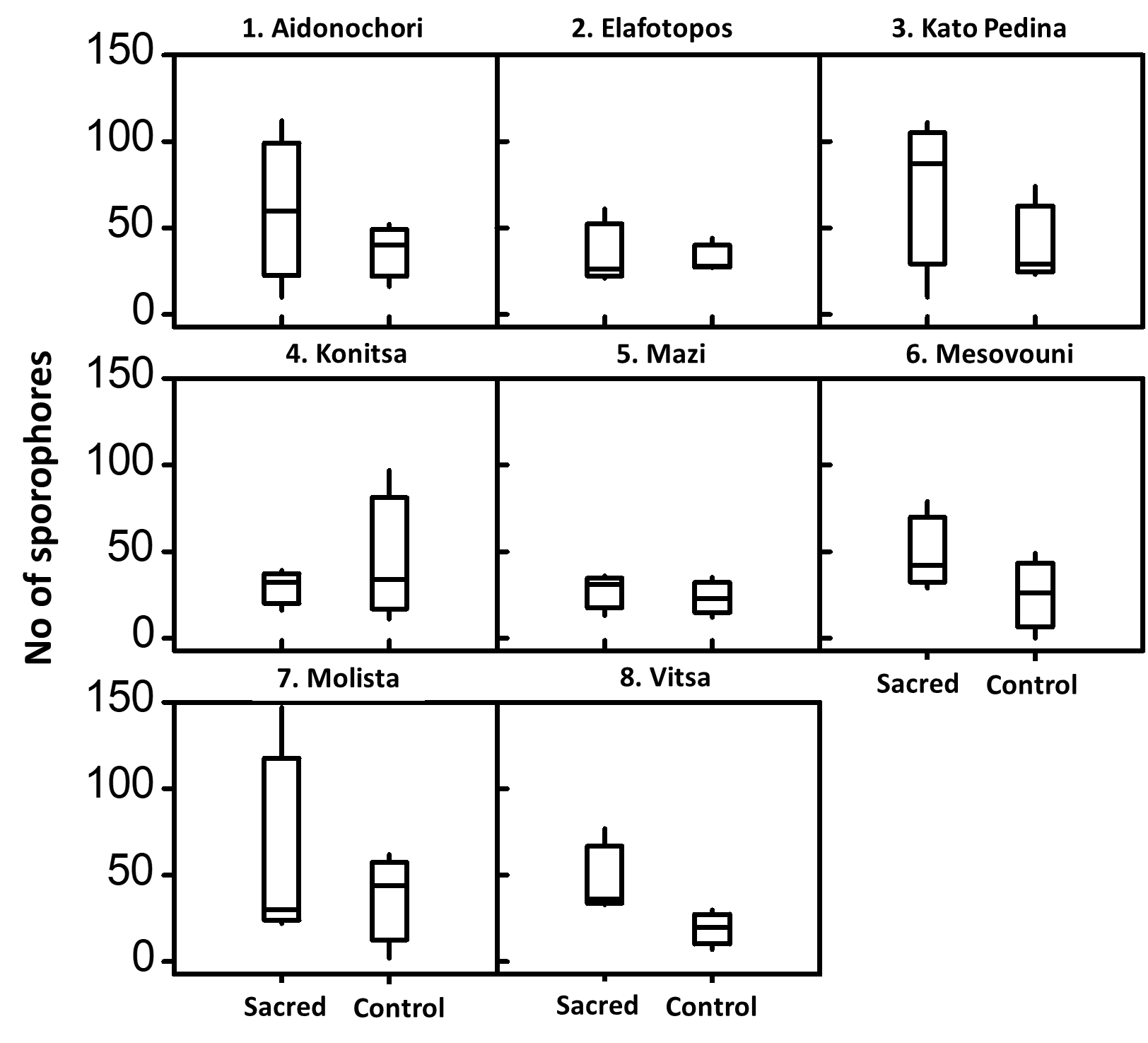
Figure 7 Distribution of sporophores across the examined sites. Boxplots show the range, medians and fungal variability (quartiles) between control and sacred plots within each examined site.
From a total of 68 ECM species, 49 were recorded in sacred plots and 39 in their matching control plots (Table 3, Figure 2). ECM incidence was higher in the control plots (managed forest) in four sites when compared with their matching sacred plots [site number (sn): 1, 3, 5, 7, Table 1] (significantly higher only in one, sn: 1), it was equal in one site (sn: 2), while it was higher in three sacred plots (sn: 4, 6, 8) (significantly higher in two, sn: 6, 8). Yield of sporophores was notably higher in five control plots (sn: 1, 2, 3, 5, 7, Table 2) while three sacred plots (sn: 4, 6. 8) showed higher figures. Yield was significantly higher in the control plots of sites 1 and 5 and also in the sacred plot of site 6. Not a single sporophore of ECM fungi was recorded in control plot of site 6.
In total 64 species of saprotrophic fungi were found, 48 of which occurred in sacred plots compared with 39 species found in managed forests (Table 4, Figure 2). The incidence was higher in sacred plots than the control plots in seven out of the eight sites examined (sn: 1, 3, 4, 5, 6, 7, 8, Table 1) (significantly higher only in sn: 7) and was equal in one site (sn: 2). The incidence did not appear higher in any of the control plots. Yield of sporophores was higher in six sacred plots compared with their matching control plots (sn: 1, 3, 5, 6, 7, 8, Table 3) (significantly higher in sn: 5, 6 and 7) and was higher (but not significantly) in two control plots (sn: 2, 4).
Out of 76 species of xylotrophic fungi that were recorded in all plots, 57 species were found in sacred sites and 42 in managed forests (Table 5, Figure 2). The incidence of xylotrophic fungi was higher in five sacred plots (sn: 1, 2, 3, 7, 8, Table 1) (significantly higher in three 3, 7 and 8). It was equal in one site (sn: 5) whereas it was higher in the control plots of two sites (sn: 4, 6) (significantly higher in sn:6). The yield of sporophores was higher in seven sacred sites (sn: 1, 2, 3, 4, 5, 7 and 8) with significant differences detected in three (sn: 1, 7 and 8).
Fungal incidence (p = 0.52, Wald test, Figure 4) and sporophore yield (p=0.4, Wald test, Figure 5) between sacred and control plots was not affected significantly by the site factor. Some differences were observed among sites (Figures 4 & 5 and Table 1) mainly due to the three way interaction (as mentioned above) of ecotrophic groups, sites and plots. It must be mentioned that higher incidence was not always followed by higher sporophore production and vice versa (Table 1).
Even though small in size, sacred groves in Greece constitute unique habitats with the main features being a) the vegetation structure due to the lack of disturbance and b) the presence of numerous gaps among large, old trees. Every single site presents a high level of variation in structure and species composition, linked to both environmental and socio-cultural drivers during past forest prohibition regimes and the absence of grazing.14 Broadleaved coppice forests are in most cases dense because of intense management by clearcut logging for firewood. Clearcut stands are relatively small (10-20 ha) so biodiversity, when considered on a regional scale, is not greatly affected by such anthropogenic intervention. Logging operations are performed in late fall and winter months and are followed by vigorous sprouting of the stumps during the following spring.
Although fungal dynamics is a key component of forest ecosystem functioning, only a few studies have dealt with fungal diversity and even fewer studies are associated with old growth woodlands in Greece. It has been thought that sacred, undisturbed woodlands act as treasuries of biodiversity. This null hypothesis was tested for fungal diversity during the current research although it was based only on rather limited 3-yr data. Even though Arnolds (1992), Barkman (1973) and Winterhoff (1992) suggested that the minimum surface of plots should range from 500 to 1,000 m², we chose smaller plots so as to have more homogeneous host-tree diversity in each plot and between corresponding pairs of sacred vs. control plots. Other researchers have also used 200 m² plots for fungal assessment23 or even smaller (100 m²).24
The ample number of 208 fungal species reported here confirms that not only sacred groves but also mixed Mediterranean ecosystems are important from a mycological viewpoint. Our results show that species richness in old-growth woods, but also in regularly clearcut coppice forests in this part of the country is particularly high, especially when considering the limited size of our plots, the low productivity of the fungal community and the limited duration of our survey.
It was found that when all site data were combined, sacred groves appeared to shelter a higher incidence of macromycete species and a higher yield of sporophores supporting the null hypothesis. When, however, the ecotrophic groups were compared individually the picture was slightly different.
Considering the ECM fungi the picture was rather ambiguous. Even though 49 species were recorded in sacred plots and 39 in their matching control plots, species richness was found to be higher in the control plots of four sites than in their matching sacred plots but only one was significantly higher. In three other sites the incidence was higher in sacred plots with the diference being significant in two of them.
The yield of ECM fungi was higher in the control plots of five sites and significantly higher in two. It was higher in the sacred plots of three sites and significantly higher in one. The results indicate that this type of Mediterranean ecosystems shelter a high fungal biodiversity regardless of the structure of the investigated forests. It is also established that vigorously growing trees with a high photosynthetic capacity tend to yield more sporophores arising from increased carbohydrate allocation to ECM fungi.25-28 Furthermore, according to Smith & Read 1997 some ECM species establish early but stop fruiting in old stands although they can persist on roots. So, it is not surprising that in our case yield was higher in young, coppice forests. As the age of all control plots was over 15 yrs old, mycorrhization of the root systems of the trees may have been little affected by clear cutting, resumed and, as a consequence, fruiting was copious. A reasonable time lag of a few years is necessary for the trees to start growing again for the mycorrhizal activity to resume.
Control plot 6-Mesovouni, was the only exception where ECM fungi, for undetermined reasons, were never recorded. Control plot 6 was abandoned agricultural land before forest trees started naturally settling. It had not been clearcut before the current study. It has been shown that the change or the end of cultivation practices in farmlands followed by afforestation lead to substantial changes in most of the physical, chemical and biological properties (contents of organic matter, nitrogen and other elements, pH, porosity, bulk density, microbial activity and the overall rate of soil processes) of soils. Previous land-use activities may have persistent effects on forest communities and their functioning, manifesting in a long-period, which may be 80–100 yrs or even longer for the recovery of forest soils.30-33 Agricultural management can reduce the abundance and diversity of soil microfungi and arbuscular mycorrhizae and affect the rate of recovery of a post-disturbed plant community.34,35 An explanation might be that in this particular site (sm: 6) more time was needed for the building up process until ECM fungi could establish.
It is also recognized that clearcut logging in coniferous forests, which regenerate naturally by seed, might create a disturbance and eventual change in the ECM community composition.36 In our case, broadleved forests resprout from the living root system of the stumps after the clear cutting operation and the only consequence expected may be the reduction in yield of sporophores for a few years. In another case in British Columbia, when studying the ECM species composition in old (75-125 yr-old) Douglas fir and birch forest vs. young (5 yr-old) plantations, it was found that from a total of 187 fungal taxa, 170 occurred in mature forests and only 17 occurred in the young plantations.37 Again, resprouting of broadleaved species after clearcut logging and the subsequent vigorous growth because of a strong pre-exising root system, as in our case, is expected to maintain the ECM composition as well as trigger profound yield of sporophores in comparison to a new plantation starting from seedlings.
Members in the family Russulaceae accounted for 41.2% of a total of 68 ECM species, The genera Inocybe and Tricholoma were found also to be dominant among ECM fungi with 11 (16,2%) and 8 (11,5%) species respectively. Our results are in accordance with Richard et al. 2004 and Zotti & Pautasso 2013 who also found the family Russulaceae to be dominant in Mediterranean holm oak (Quercus ilex) forests in Corsica and Liguria, Italy, rspectively. Six species, Cortinarius bivelus, Tricholoma lascivum, Melanoleuca rasilis, Ampelomyces quisqualis, Anthostoma acerinum and Ramularia endophylla represent new recordings in Greece. Those are marked with an asterisk in Tables 3, 4, 5 and 6.
Incidence and yield of saprotrophic fungi were higher in sacred plots compared with their matching control plots. Similar results in old-growth holm oak (Quercus ilex) forests in Corsica were attributed to the accumulation of suitable and varied substrates but also to the existing gaps in the canopy.37
In the group of xylotrophic fungi, incidence and yield were evidently higher in sacred plots than in managed forest. Ιn the control plots most xylotrophic species were growing on old stumps, while in sacred plots, where only a few stumps existed, most of the sporophores grew on coarse woody debris and old standing trees. Stumps hold more moisture and for a longer period of time in dry climates like Greece thus they are colonized by xylotrophic fungi more profoundly. Daedaleopsis nitida, a rare species in Greece, was found on dead branches of Quercus frainetto in the sacred plot of Vitsa (8). A cluster of 7 sporophores of Daldinia concentrica, another rare species, was found on Carpinus orientalis in the control plot of Mesovouni (6).
Fungi have not been considered for conservation in Greece so far. Results generated by research projects such as the current one may be of crucial importance for initiating further, similar research, but also in emphasizing the need for conservation measures. Protecting old-growth patches such as sacred groves or, even better, entire forest compartments, will promote and assure the conservation of fungi, especially xylotrophic and saprotrophic. On the other hand, the high species richness of ECM species encountered in managed forests illuminates the important role this group of fungi plays in the health and stability of the Mediterranean ecosystems which grow under climatic stress and, in addition, they have been under exploitation for centuries. Thus, it may be interesting to monitor ECM fungi in managed forests and in abandoned farmland during the afforestation process in order to evaluate the ecosystem health.40
Further investigation is required to better characterize the mycodiversity of sacred groves in comparison to managed forest where the intensity and types of human activities may affect future conservation strategies.
On the basis of the current research the following conclusions were reached:
1) sacred, undisturbed sites hold higher fungal diversity of saprotrophic and xylotrophic fungi because of the old-growth trees they hold and consequently, the substrata and habitats are more suitable,
2) the group of ECM fungi shows a remarkable incidence in both sacred groves and managed coppice forests but the yield was definitely found higher in managed forests, and
3) although broadleaved coppice forests in the study area are usually managed by clearcut logging every 30 yrs, fungal diversity still appears to be satisfactorily high.
None.
The authors declare no conflict of interest.

©2021 Diamandis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.