Journal of
eISSN: 2373-437X


Review Article Volume 11 Issue 5
Preventive Conservation laboratory, National Archive of the Republic of Cuba, Cuba
Correspondence: Sofia Borrego, Preventive Conservation laboratory, National Archive of the Republic of Cuba, Compostela No. 906 esq a San Isidro, Habana Vieja, PO Box: 10100, Havana city, Cuba, Tel (53) 7862-9436, Fax (53) 7866 8089
Received: November 23, 2023 | Published: December 29, 2023
Citation: Borrego S. Fungal diversity in environments of repository of the national archive of the Republic of Cuba from the 80s to 2022. J Microbiol Exp. 2023;11(5):156-166. DOI: 10.15406/jmen.2023.11.00404
This work aims to perform a compilation of the fungal diversity in the environment of the National Archive of the Republic of Cuba (NARC) repositories from the 80s of the last century and until 2022, which includes the fungal genera and species isolated from: air, settled dust and document surface, as ecological niches that contribute to the environmental mycological quality of these spaces in the institution. A total of 55 references were analyzed (31 of air, 4 of settled dust and 20 of different types of documents surface). A total of 168 fungal genera and 54 species were isolated from air, 36 genera and 12 species from dust as well as 98 genera and 26 species were detected on the document surfaces with a marked predominance of the genera Aspergillus, Cladosporium and Penicillium in the three niches, although 24 rare genera were detected in air after the year 2000 (e.g. Acremonium, Aureobasidium, Beltraniella, Bipolaris, Blastomyces, Botryoderma, Botryotrichum, Chaetomium, Chrysonilia, Exophiala, Gilmaniella, Harposporium, Hyalodendriella, Itersonilia, Nodulisporium, Ovulariopsis, Papularia, Scolecobasidium, Sepedonium, Sprorobolomyces, Torula, Trichophyton, Wardomyces, Zygosporium). Only six genera were similarly isolated from air, dust and the document surfaces (9.7%), while 19 species were found in these three niches (9.5%) (e.g. A. flavus, A. niger, A. versicolor, Cl. caryigenum, Cl. cladosporioides, Cl. herbarum, P. chrysogenum, P. citrinum, P. commune, P. janczewskii). Although fungal isolations from dust and the document surfaces are still scarce, it is evident that the greatest diversity of species was isolated from the air and that the similarities of species between these three niches are low, therefore air is the matrix that contributes with the most fungal species to the NARC environment. This compilation of the fungal diversity in the environment of the NARC repositories will serve as a reference for future studies in Cuban archives and other countries.
Keywords: fungal diversity, archive, air, dust, document surface, indoor environment, environmental mycological quality
NARC, National Archive of the Republic of Cuba; T, temperature; RH, relative humidity; RF, relative frequency; NSM, non-sporulating mycelia
The archives, libraries and museums are the institutions that preserve the legacy of humanity. They contain many documents of heritage value written on various media (papyrus, parchment, paper, etc.), and they keep other types of documents such as photographs, maps and plans, engravings as well as digital documents, among others. These organic, inorganic and/or synthetic materials deteriorate over time, but this process is accelerated by the effect of physical (light, temperature [T], relative humidity [RH]), chemical (atmospheric pollution) and biological agents (microorganisms, insects).1
The knowledge of the continuous control of environmental conditions in archives, libraries and museums currently constitutes one of the most important elements to consider in the preventive conservation of a nation's documentary heritage. The prevalence of inadequate environmental conditions together with the presence of high microbial concentrations in the repositories environment where this heritage is preserved has increasingly awakened the attention of researchers and specialists in the area of the heritage assets conservation, due to the risk that this represents both for the heritage integrity they preserve and for the staff health who work in these institutions or receive systematic services in them.2−4 Particularly, fungal contamination is one of the main objects of study, since fungi constitute the largest group of biological agents that are transported by air and dust, in addition to this microbial group having high biodeteriogenic5−9 and pathogenic10−17 potentialities.
The dust particles composition varies in quantity and quality depending on the building location, the activities carried out inside it, the year season and the conservation conditions of documents, books, and objects.18,19 When it deposited on the collections, create micro-environmental conditions on their surfaces that prevent normal air flow, facilitating water to be absorbed by materials and constitute a nutritional source that promotes the triggering of fungal growth20 that it can form extensive biofilms on documents, objects, and different surfaces. On the other hand, it is known that papers and other documentary materials become infected during the manufacturing process21,22 and that the fungi existing inside them can trigger biodeterioration of these supports when the environmental conditions are optimal for their growth and propagation since they use all their components as nutrients.
The existence of high values of T and RH in countries with a tropical climate, such as Cuba, favors the increase of load dust and the fungal propagules (spores, hyphal fragments, partial conidiophores, pieces of mycelium, sclerotia) concentration in the air, as well as their deposition on different materials, facilitating their development and proliferation. They have powerful, versatile and adaptable metabolic machinery, which allows them to degrade a great diversity of substrates, both of organic and inorganic origin, promoting the biodeterioration of the different materials stored in the repositories of archives and libraries.2,6,8,23−27 These effects are greater in countries with a tropical climate where pests that are difficult to control and eliminate can sometimes be unleashed. Likewise, fungi are characterized by having different structures and pathogenicity mechanisms, which cause allergies and other specific conditions in humans.15,28−31
Numerous studies have established a close relationship between environmental conditions, the presence of viable fungal propagules in the environment or inside the supports and their incidence on triggering of biodeterioration in heritage works32−34 and respiratory conditions in humans,28,31,35,36 managing to associate their presence with the development of symptoms typical to these pathologies and other types of diseases.11,13,15 For this reason, multiple research groups recommend the need to increase the systematic studies frequency of the environmental conditions in the premises to evaluate the environments quality, in order to guarantee an environmental characterization of the same that allows for the early solution of problems associated with the outbreak of pests and the staff health effects.
The indoor environments of archives and libraries are a reservoir of fungal propagules due mainly to the abundance of dust, the heterogeneous nature of the substrates and the conditions of overcrowding of documents in the repositories, which is why they constitute complex ecosystems.37,31 In the National Archive of the Republic of Cuba (NARC), since the 1980s, research began on environmental microbiological quality and the documentary materials biodeterioration caused mainly by fungi. Since then, studies have been carried out that have yielded multiple results that have been presented at events and published in national and international journals, and these topics have also contributed to the training of graduates in Microbiology since there have been various theses that have been supervised. Taking these elements into account, the aim of this work was to perform a compilation of the fungal diversity in the environment of the NARC repositories from the 80s to the 2022, which includes the fungal genera and species isolated from air, settled dust and the surfaces of documents as ecological niches that contribute to the environmental mycological quality of these spaces in the institution.
A search was performed for reports in publications, presentations at events and bachelor's thesis in Microbiology that refer to the studies that were carried out in the NARC from the years 1985 to 2022 of the mycobiota detected in three niches that they were: the indoor air of the repositories, the documents surface as well as the dust settled on the shelf, the ventilation ducts or in collectors.
In the air mycobiota reports, the obtained results of both passive and active methods were considered, that is, using biocollectors. For the settled dust, the information from studies that were carried out with both the dust that settled in collectors and the dust that was collected from the furniture and/or documents surface by aspiration was considered. In the analysis of the mycobiota isolated from documents surface, data were taken from studies on paper, on bindings and on other materials such as graphic techniques (mainly on plant leaves) and photographs on different materials (paper, textile, inorganic) as well as different techniques of maps and plans on paper and textile. Likewise, presentations at national and international conferences, several bachelor's and master's theses in Microbiology were considered based on studies carried out at the NARC and other Cuban institutions that also preserve the documentary heritage. These documents are conserved in the NARC library and in the Preventive Conservation laboratory itself, as well as in Biology Faculty of the Havana University. The other international information reviewed appeared in different databases as Biological Abstract, Chemical Abstract, CIRC, Emerging Sources Citation Index (ESCI), Google Scholar, Latindex, PubMed, REDIB, Redilac, ResearchGate, Scielo, Science Direct, Scopus, etc. Several terms and their combinations were used to carry out the searches, which were: fungi, molds, microorganisms, paper, archive, library, air, airborne fungi, dustborne fungi, settled dust, fungi on documents surface, books, museum, documentary heritage, cultural heritage and health.
Ecological classification of genera and species
Relative frequency (RF) of the fungal genera and species detected in the three niches studied was determined according Esquivel et al38 where
RF = (Reports number where a genus or species is detected / Total number of analyzed reports by niche) x 100
The ecological categories are classified as: Abundant (A) with RF = 100-81%, Common (C) with RF = 80-61%, Frequent (F) with RF = 60-41%, Occasional (O) with RF = 40-21%, Rare (R) with RF = 20-0%.34
Diversity of fungal genera and species reported in the repositories air
Most environmental studies conducted at NARC focused on aerial mycobiota. A total of 31 references were detected that reported the different genera isolated from the air and of them 23 that also mentioned the detected species (Figure 1). In the air studies performed, two types of sampling methods were mainly used, one passive (Omeliansky)2,23,36,39−44 and the other active. In this last method, two types of biocollectors were used, one with slits (Chirana aeroscope)45 and another with an orifice (SAS collector).34,36,46−52 A total of 54 genera, 168 species and two types of non-sporulating mycelia were reported for the air, one of them white and the other pigmented (Figure 2).
Despite the difference in methods used, during the years analyzed, a wide diversity of fungal genera belonging fundamentally to the phylum Ascomycota has been obtained, which is characteristic of indoor environments,53 with a marked predominance of the genera Aspergillus, Cladosporium and Penicillium, that were seconded by Alternaria and Fusarium (Figure 3A). The marked prevalence of Aspergillus, Cladosporium and Penicillium in all environments classifies them from an ecological point of view as abundant genera, the same occurs with Alternaria and Fusarium. This result coincides with previous studies performed in archive, library and museum environments by other Cuban authors54−63 and foreigners.20,64−77 However, the genera Aspergillus and Penicillium were not detected in a study conducted in an archive, library and museum of Italy by Ruga et al.33
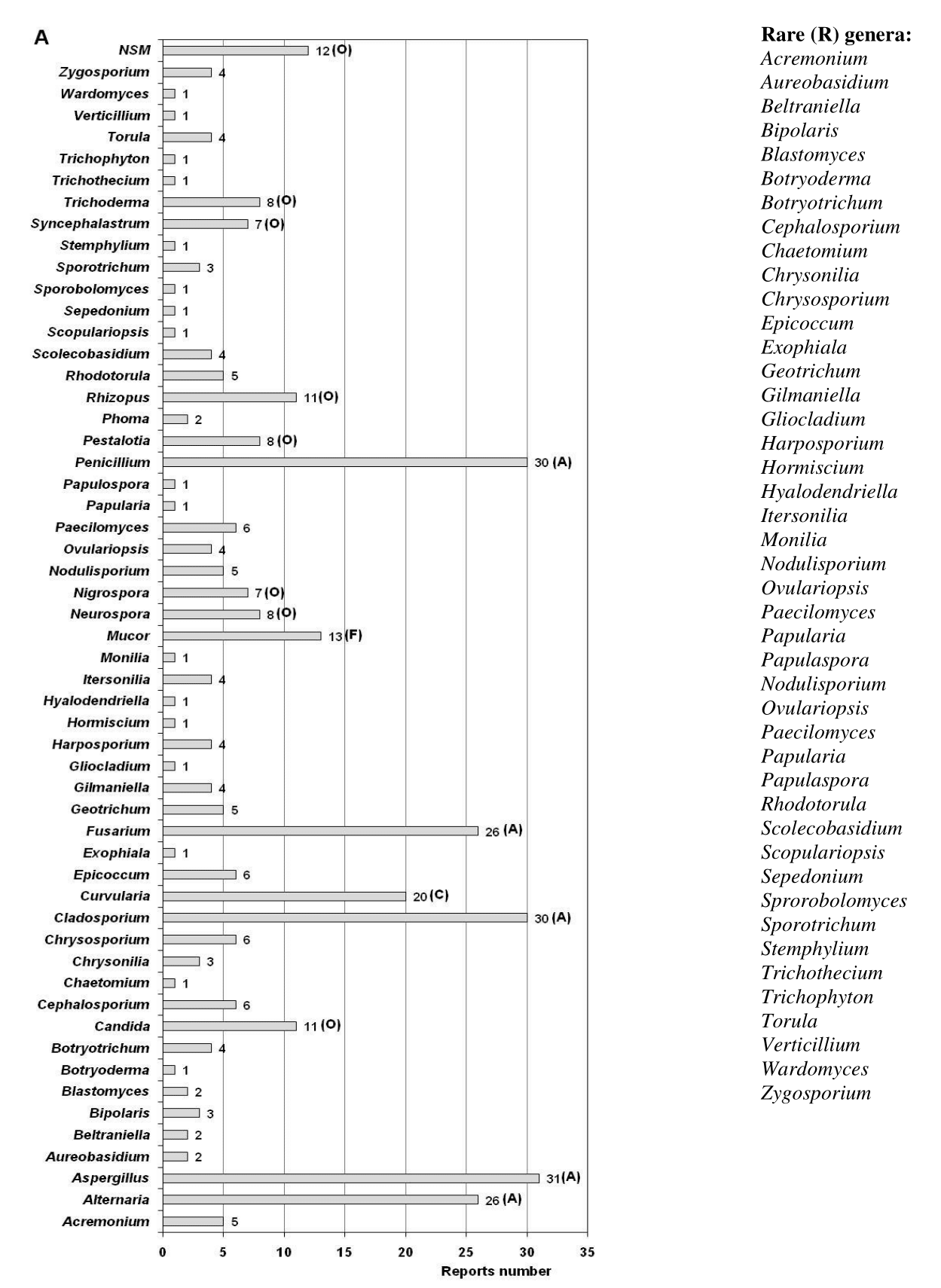
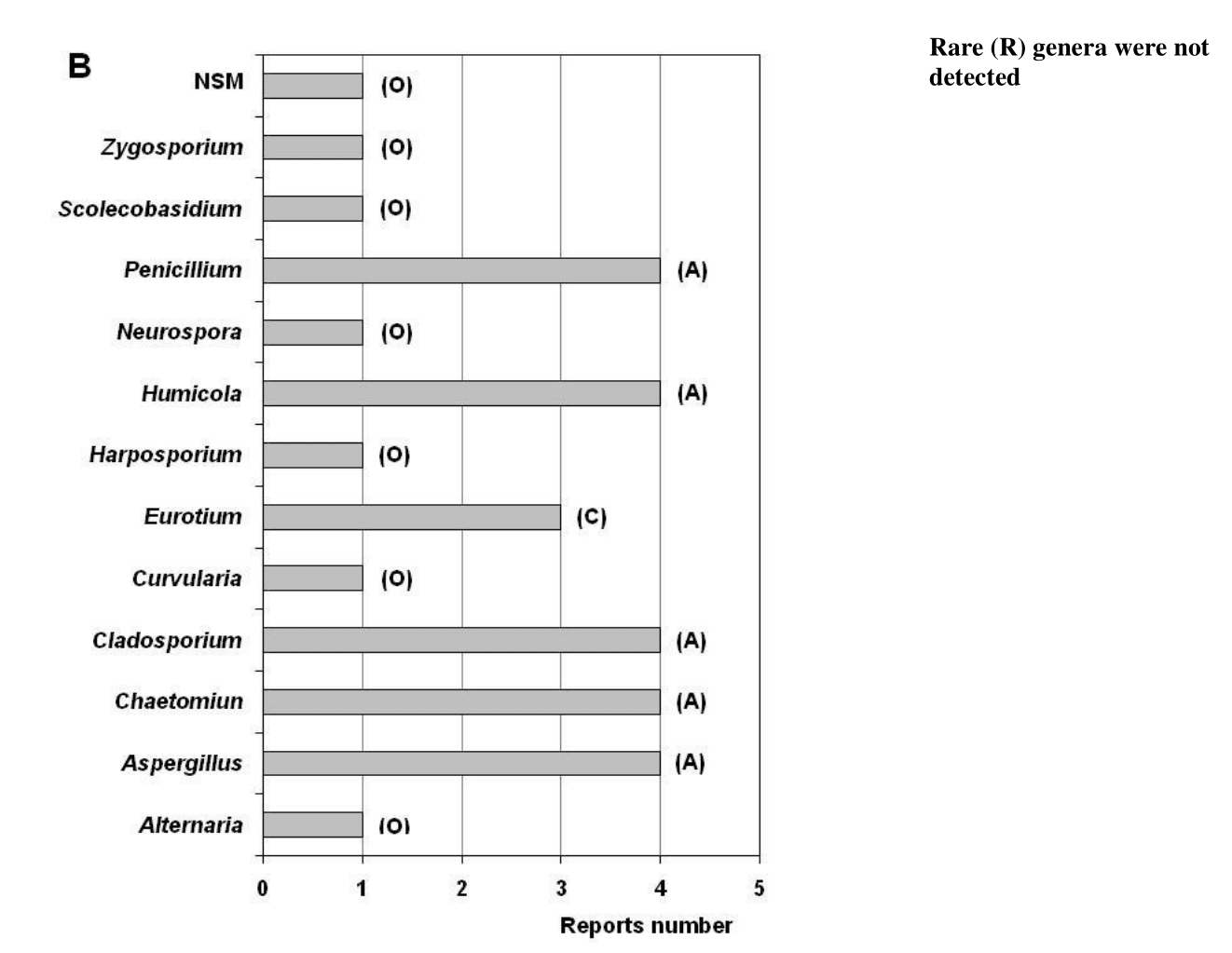
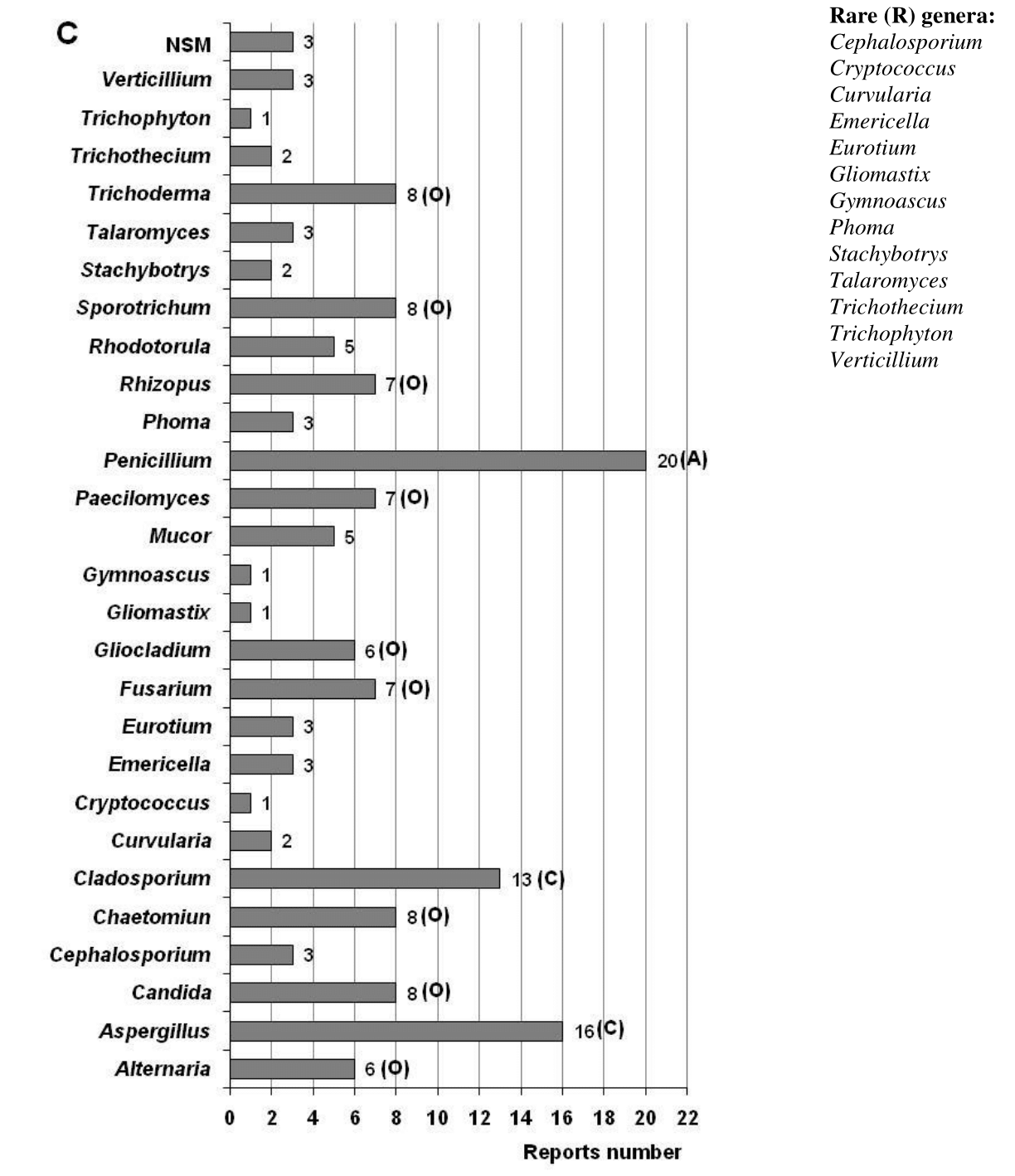
Figure 3 Ecological behavior of the genera detected in the air (A), in settled dust (B) and on the documents surface (C). The total reports number analyzed for the air was 31, for the settled dust it was 4 and for the documents surface it was 20, each equivalent to a RF of 100%. Ecological categories: Abundant (A) with RF = 100 – 81%; Common (C) with RF = 80 – 61%; Frequent (F) with RF = 60 – 41%; Occasional (O) with RF = 40 –21%; Rare (R) with RF= 20 – 0.1%. NSM: Indicates non-sporulating mycelia.
On the other hand, Curvularia was a genus classified as common, while Mucor was categorized as frequent. Others such as Candida, Neurospora, Nigrospora, Pestalotia, Rhizopus, Syncephalastrum and Trichoderma as well as the non-sporulating mycelia were classified as occasional, but 40 genera were classified as rare and among them found: Acremonium, Aureobasidium, Beltraniella, Blastomyces, Cephalosporium, Chaetomium, Exophiala, Gilmaniella, Harposporium, Hormiscium, Ovulariopsis, Papularia, Papulaspora, Sepedonium, Sprorobolomyces, Verticillium, Wardomyces, Zygosporium, etc. In total, 5 genera were classified as abundant, 1 common, 2 frequent, 7 occasional, and 40 rare (Figure 4A). The genera Curvularia, Mucor, Nigrospora, Rhizopus, Syncephalastrum and Trichoderma have been detected in the atmosphere of Havana,78−81 which suggests that they could have penetrated the indoor air of the repositories and have remained in these environments.
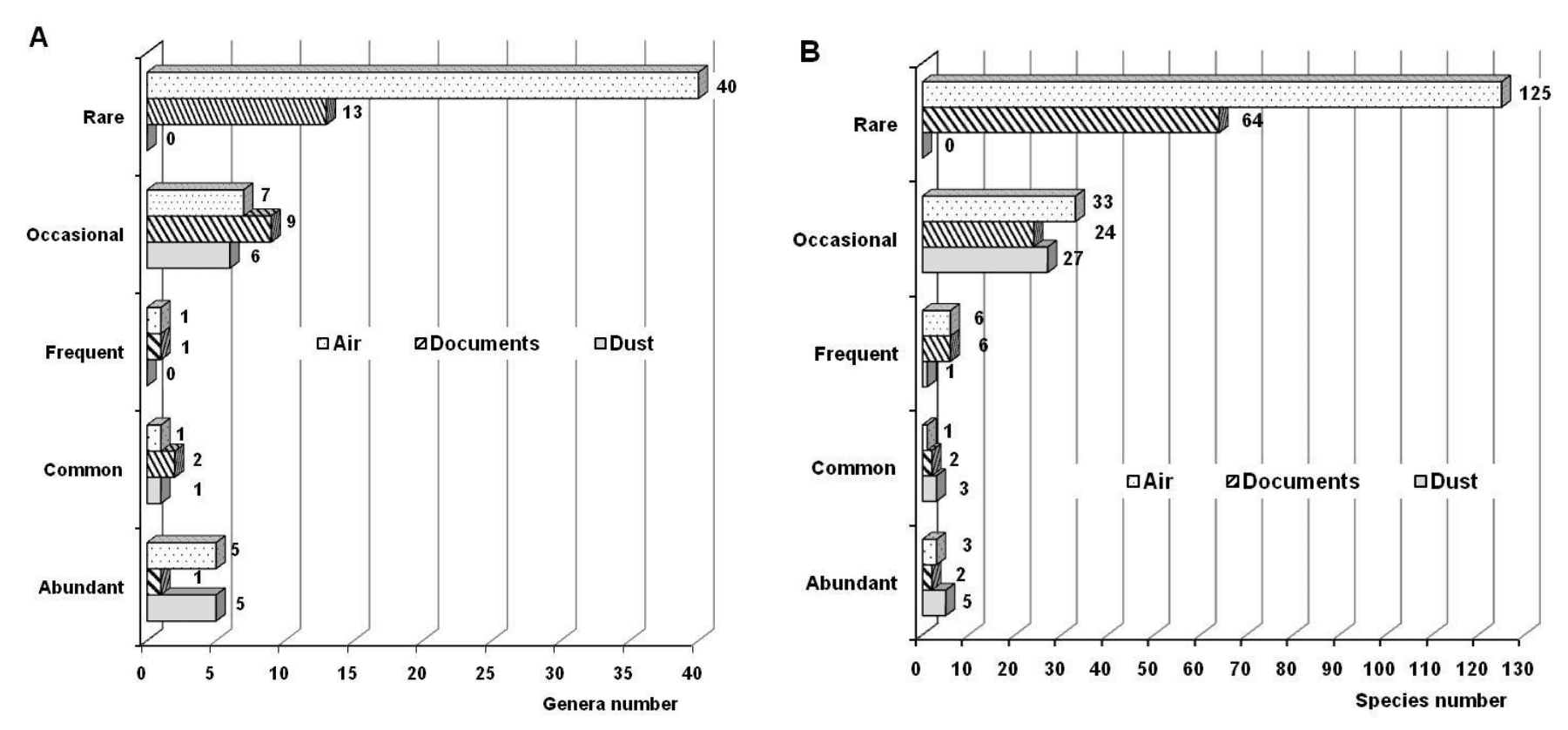
Figure 4 Ecological behavior of the different genera (A) and species (B) isolated in the three studied niches.
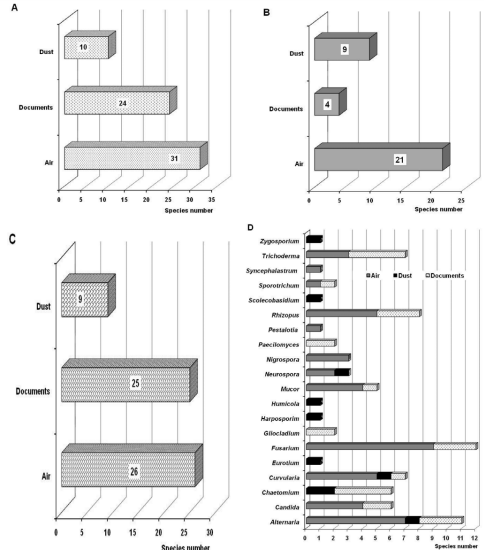
Figure 5 Species number of the Aspergillus (A), Cladosporium (B), Penicillium (C) genera and other detected genera that were classified as abundant, common, frequent and occasional (D).
Within these rare genera, some were only reported before the year 2000, such as Gliocladium, Hormiscium, Monilia, Papulaspora, Phoma, Scopulariopsis, Sporotrichum, Stemphylium, Trichothecium and Verticillium, while others were first reported after that year. However, some of these genera were previously isolated from the air of archives, libraries and museums in other countries, such as Gliocladium, Papularia, Phoma, Scopulariopsis, Stemphylium, Trichothecium and Verticillium.67,70,72,75,82−90
The rare genera found after the year 2000 were Acremonium, Aureobasidium, Beltraniella, Bipolaris, Blastomyces, Botryoderma, Botryotrichum, Chaetomium, Chrysonilia, Exophiala, Gilmaniella, Harposporium, Hyalodendriella, Itersonilia, Nigrospora, Nodulisporium, Ovulariopsis, Papularia, Scolecobasidium, Sepedonium, Sprorobolomyces, Torula, Trichophyton, Wardomyces and Zygosporium. Of them, Aureobasidium, Botryoderma, Chaetomium, Chrysonilia, Nodulisporium and Zygosporium were previously isolated from the air of Cuban libraries and museums, some of them naturally ventilated,54,91,92 which demonstrates that although in low concentrations, these genera can be found in the outdoor air. The genera Acremonium, Aureobasidium, Chaetomium, Chrysonilia, Exophiala, Trichophyton and Torula have been detected in the air of foreign archives, libraries and museums.32,37,64,67,70,72,73,77,85,86−88,90 Likewise, the genera Itersonilia and Sprorobolomyces were isolated from dust settled on books in an Italian library93 and a member of Botryotrichum genus was isolated from the documents surface preserved in a Polish library.94 On the other hand, the genera Aureobasidium, Bipolaris, Sepedonium, Torula and Trichophyton were found in repositories of the Provincial Historical Archive of Pinar del Río (located in the westernmost province of the Cuba),60 while the genera Torula and Zygosporium were found in the air of repositories of the Provincial Historical Archive of Santiago de Cuba (one of the eastern provinces of the country).62 Both archives are characterized by having natural ventilation in their repositories, which suggest that these genera can be in the outdoor air and penetrate the archive environment through the open windows and the natural ventilation systems. However, Beltraniella, Blastomyces, Gilmaniella, Harposporium, Hyalodendriella, Ovulariopsis and Wardomyces genera have only been reported for the NARC36,47,48,50−52 and have not been found in the bibliography consulted for archive, library, and museum environments in other countries.
Beltraniella, Gilmaniella, Nodulisporium and Wardomyces are genera that could also have been found in the outdoor air and that penetrated the NARC repositories at some point through the natural ventilation system they have, since they were previously detected in the outdoor air the NARC51 and in a Cuban museum that is located near the NARC.91
Blastomyces genus is characterized by being a dimorphic fungus capable of growing in a saprophytic mycelial form at 25°C and in a pathogenic yeast-like form at 37°C; and it is considered an emerging pathogen.11 It has two species, B. dermatitidis and B. gilchristii, which are morphologically identical but distinguishable by sequence analysis of the ITS region.95 This fungus lives in the soil and is associated with the decomposition of organic matter such as leaves and wood. It is the causal agent of blastomycosis96 and has been detected in bone conditions15 so it is considered risky for human health, hence it belongs to the species group of biohazard III.97 This genus has been frequently detected in North America where blastomycosis is endemic.16 The genus Sepedonium is known to be a saprophytic fungus that grows in soil and on plant material98 but has been isolated from the indoor air of homes in Nigeria,99 from Iranian hospital rooms100 as well as the paper and parchment that make up books.101 It is a fungus that can cause infections in humans, particularly in immunocompromised people.102,103 Trichophyton is a dermatophyte fungus, i.e., it causes superficial mycosis affecting the skin, nails and hair. It can be found on the land, and although its fundamental transmission is through the air, it can be transferred by people.104 Despite being a rare genus in the air of archives, libraries and museums, it was recently found in the air of a Cuban archive60 and in an Italian museum.77 These fungi belonging to the three aforementioned genera could have entered the air of the repositories through the natural ventilation they have, since the NARC is surrounded by soil, vegetation, streets and avenues with high pedestrian and vehicular traffic, natural niches where they can be found.
Although Aureobasidium was isolated from the air of NARC repositories for the first time before 2000,105 it was found again in 201647,48 so it turns out to be a rare genus. However, it has been found in the environment of a Polish archive,82 in the air of several French archives,84 in an Italian library,94 in a Greek library72 and from an Indonesian library.70 This black yeast can be found in settled dust,106 as it is characterized by growing in extreme environments.107 Some species, such as A. pullulans and A. melanogenum, can be opportunistic pathogens and cause asthma and other conditions in humans.17,107,108
Of the 168 species isolated from the air, 31 corresponded to Aspergillus genus (18.5%), 21 to Cladosporium (12.5%) and 26 to Penicillium (15.6%); the remaining 53.4% corresponded to other genera (Figure 5). Within Aspergillus, two species were classified as abundant (A. flavus, A. niger), one turned out to be frequent (A. versicolor), 12 were classified as occasional (Aspergillus sp., A. alliaceus, A. candidus, A. clavatus, A. flavipes, A. fumigatus, A. glaucus, A. japonicus, A. nidulans, A. ochraceus, A. oryzae, A. terreus) and 16 turned out to be rare species (A. athecius, A. auricomus, A. clavatoflavus, A. conicus, A. ellipticus, A. ornatus, A. ostianus, A. panamensis, A. parasiticus, A. penicillioides, A. restrictus, A. roseus, A. sydowii, A. tubingensis, A. unguis, A. unilateralis) (Table 1). The species A. flavus and A. niger have been isolated with prevalence in foreign archives, libraries, and museums.37,66,67,73,74,109−111 From Cladosporium genus, no species was found to be abundant. However, only one species was classified as common (Cl. cladosporioides), six were occasional (Cladosporium sp., Cl. elatum, Cl. fulvum, Cl. herbarum) and 15 were classified as rare (Cl. allii-porri, Cl. avellanium, Cl. basiinflatum, Cl. caryigenum, Cl. coralloides, Cl. cucumerinum, Cl. epiphyllum, Cl. gossypiicola, Cl. lignicola, Cl. macrocarpum, Cl. minourae, Cl. staurophorum, Cl. subuliforme, Cl. tenuissimum , Cl. werneckii). Of this genus, the species Cl. cladosporioides and Cl. herbarum have been detected more frequently37,67,82 than others, such as for example Cl. elatum, Cl. macrocarpum and Cl. tenuissimum.37 From Penicillium genus, no species was detected that was classified as abundant, but two species were categorized as frequent (Penicillium sp., P. citrinum), four as occasional (P. chrysogenum, P. citrorosum, P. glaucum, P. griseofulvum) and 20 rare. Within this genus, the species P. chrysogenum was predominant in a Turkish library67 and in Polish archives and libraries,82 while P. citrinum was dominant in Brazilian libraries.37 It is noteworthy that the species A. flavus, A. niger, Cl. cladosporioides, P. citrinum and P. chrysogenum have been detected with high frequency from the atmosphere of Havana78,81 which explains its noticeable presence in the indoor environment of the NARC repositories. On the other hand, many of these species are allergenic, toxigenic and can cause superficial and deep mycoses, therefore they are considered primary pathogens and/or opportunistic pathogens.12−14,29,112,113
Regarding the general behavior of the species, a predominance of three species (Alternaria sp., Aspergillus flavus, Aspergillus niger) was observed, which were classified as abundant (Table 1). Only the species Cl. cladosporioides was categorized as common, six species were classified as frequent (A. versicolor, Cladosporium sp., Curvularia sp., Fusarium sp., Penicillium sp., P. citrinum) and 33 were classified as occasional (Alternaria geophila, Aspergillus sp., A. alliaceus, A. candidus, A. clavatus, A. flavipes, A. fumigatus, A. glaucus, A. japonicus, A. nidulans, A. ochraceus, A. oryzae, A. terreus, Candida sp., Cephalosporium sp., Chrysosporium sp., Cladosporium elatum, Cl. fulvum, Cl. herbarum, Cl. oxysporum, Cl. sphaerospermum, Curvularia lunata, Epicoccum sp., Fusarium solani, Geotrichum candidum, Mucor racemosus, Penicillium chrysogenum, P. citrorosum, P. glaucum, P. griseofulvum, Pestalotia sp., Rhizopus oryzae, Trichoderma sp.). The remaining 125 species were classified as rare (Figure 4B). From rare species, some were detected before the year 2000 while others were isolated later. Several of the species of Aspergillus, Cladosporium and Penicillium that were found to be abundant (A. flavus, A. niger), common (Cl. cladosporioides), frequent (A. versicolor, P. citrinum), occasional (A. alliaceus, A. candidus, A. clavatus, A. flavipes, A. fumigatus, A. glaucus, A. nidulans, A. japonicus, A. ochraceus, Cl. elatum, Cl. herbarum, Cl. sphaerospermum, P. chrysogenum, P. griseofulvum) and rare (A. ostianus, A. parasiticus, A. sydowii, Cl. macrocarpum, Cl. tenuissimum, P. digitatum, P. implicatum, P. oxalicum, P. simplicissimum), were reported in Brazilian library environments by Leite Jr. et al.37,109 Likewise, some Aspergillus species isolated in the NARC environment (A. candidus, A. flavus, A. glaucus, A. niger, A. ochraceus, A. versicolor) were recently detected in a Colombian library,114 and many of the species of these three genera have been reported by other authors in archive and library of other countries,66,67,74,82,84 as well as in museums.73
Among the rare species detected before the year 2000 is Cladosporium werneckii Horta, which was reported by Vaillant et al.115 It has been known that it was later classified as Hortaea werneckii (Horta) Nishim. & Miyaj,116 and can also grow in environments with high concentrations of NaCl (up to 30%) so it is a halophilic species.117,118 Likewise, this species can cause superficial mycosis in humans since it is the causal agent of black tinea, a typical colonization of the hands and feet in combination with hyperhidrosis.10,117,119,120
It should be noted that as of 2010, mycelia that were described as sterile or non-sporulating mycelia began to be detected in the NARC air. As these mycelia have been isolating more frequently from the air of this archive, they have been ecologically categorized as frequent. In other Cuban archives, these type of mycelia has also been detected, and fundamentally referring to two types of them, one is white and another is pigmented.51,55,60−63,121 These mycelia have been isolated to different concentrations and are still detected in the environment of the NARC repositories as shown in this study. It is noteworthy that it has been discovered that mycelia of these types may be non-sporulating forms of pathogenic fungi, so they may be potential emerging pathogens.122
Variety of genera and species reported in the dust collected
In total, four reports made from 2012 until 2022 were analyzed demonstrating the low frequency of studies in this niche (Figure 1). From the dust has been isolated a total of 12 genera and 36 species typical of the Ascomycota phylum (Figure 2). Five genera turned out to be abundant (Aspergillus, Chaetomium, Cladosporium, Humicola, Penicillium), one was classified as common (Eurotium) and six turned out to be occasional (Alternative, Curvularia, Harposporium, Neurospora, Scolecobasidium, Zygosporium) (Figure 3B). Genera cataloged as rare were not detected in dust. The presence of non-sporulating mycelia in this niche is also reported.
As can be seen again in this niche, the genera Aspergillus, Cladosporium and Penicillium were predominant, followed by Chaetomium and Humicola. The prevalence of the first three mentioned genera is because they can remain viable and grow in various habitats and under dissimilar environmental stress conditions.20 The presence of various species of Aspergillus and Penicillium in home dust has been reported in different regions of the planet.123,124 The prevalence of Aspergillus, Cladosporium and Penicillium genera was also detected in a study that analyzed the housing dust located in Havana125 and from systems of air conditioning units in a Chinese museum.126 Alternaria, Chaetomium and Curvularia genera as well as the non-sporulating mycelia both hyalines and pigmented were also found in dust collected in Havana homes.125
Alternaria, Aspergillus, Chaetomium, Cladosporium, Eurotium and Penicillium genera as well as sterile or non-sporulating mycelia have previously been found in dust collected in archives. In the same way, these genera have been isolated from other environments such as museums, libraries, offices, homes and schools.19,106,126−129,130,131 Humicola genus has previously been isolated from the archives, libraries and museums air,54,77,82,86,89 from the indoor dust of buildings with humidity132 as well as from the lenses of deteriorated binocular preserved in a Vietnamese museum,133 and can cause illness in humans, particularly in immunocompromised people.134
In general, of the 36 species isolated from dust, five were classified as abundant (Aspergillus flavus, A. niger, Cladosporium caryigenum, Cl. cladosporioides, Humicola sp.), three as common (Aspergillus chevalieri, Chaetomium sp., Penicillium janczewskii), one as frequent (Penicillium sp.) and the remaining 27 species were classified as occasional (Figure 4B, Table 1). It should be noted that no species was categorized as rare for this niche.
Of the species isolated from the dust, 10 correspond to the Aspergillus genus (26.3%), nine to Cladosporium and Penicillium (23.7%), respectively. The remaining 26.3% matched to species from other genera (Figure 5). Of the Aspergillus species, two were found to be abundant (A. flavus, A. niger) and the remaining eight species were occasional (A. chevalieri, A. clavatus, A. glaucus, A. oryzae, A. penicillioides, A. restrictus, A. terreus, A. versicolor). Two species of Cladosporium were classified as abundant (Cl. caryigenum, Cl. cladosporioides) and the remaining seven were classified as occasional (Cl. basiinflatum, Cl. elatum, Cl. herbarum, Cl. hillianum, Cl. oxysporum, Cl. sphaerospermum, Cl. tenuissimum). It should be noted that for these two genera only abundant and occasional species were found, e.i, no species were detected that could be classified as common, frequent, or rare. In the case of the Penicillium genus, one common species was found (P. janczewskii), one was classified as frequent (Penicillium sp.) and seven as occasional (P. aurantiogriseum, P. chrysogenum, P. citrinum, P. commune, P. decumbens, P. digitatum, P. simplicissimum). For this genus, neither abundant nor rare species were detected.
Although there are few studies on the microbiological characterization of dust, whether collected or settled on the surfaces (including the documents surfaces) in archives, libraries and museums, a wide diversity of fungal species is reported in this niche. However, Aspergillus species have always been present with good representation37,93 and within them A. flavus and A. niger are the most abundant, therefore the most mentioned,37,73 the same occurs for dust from homes.135 Laxmana-Chary,131 in a study carried out in libraries in India, found the species A. flavus, A. versicolor, Cl. herbarum and P. citrinum in the dust, as well as sterile mycelium, independently of other species belonging to other genera.
Diversity of genera and species detected on the documents surface
A total of 20 references reporting fungal isolations from the surface of different types of documents were analyzed (Figure 1). A total of 26 genera, 98 species and three non-sporulating mycelia are reported (Figure 2) with a predominance of the phylum Ascomycota, coinciding with previous reports.7,9,26,93,136−138 From genera found, only Penicillium turned out to be abundant (Figure 3C). Aspergillus and Cladosporium were common genera, Candida was found to be frequent, and nine genera were classified as occasional (Alternaria, Chaetomium, Fusarium, Gliocladium, Mucor, Paecilomyces, Rhizopus, Sporotrichum, Trichoderma). The remaining 13 genera were categorized as rare.
Mesquita et al.139 identified and characterized the mycobiota isolated from historical documents preserved in the archive of the Coimbra University in Portugal, and of the 14 detected genera Aspergillus, Cladosporium and Penicillium prevailed. Likewise, other authors have referred to these genera along with Chaetomium and Trichoderma as common mycobiota in ancient documents.140−143 Aspergillus and Penicillium were referred to recent works as predominant genera,9,26 while others authors cite the Cladosporium genus144 and the Penicillium genus145 as dominants. Genera Chaetomium, Fusarium, Paecilomyces and Rhizopus were isolated from books and documents from the library of Istanbul University,67 while Alternaria, Chaetomium, Fusarium and Trichoderma were previously detected in deteriorated historical books and documents.136,146 Likewise, representatives of the genera Alternaria, Aspergillus, Candida, Chaetomium, Cladosporium, Mucor, Paecilomyces, Penicillium, Rhizopus, Sporotrichum, Stachybotrys and Trichoderma were found in Polish documents.26,147 Recently, different species of the genera Alternaria, Aspergillus, Chaetomium, Cladosporium, Eurotium, Fusarium, Penicillium and Talaromyces were detected from the surface of photographs and audiovisual media.27,137,148
Among the genera considered as rare are Cryptococcus, Mucor and Rhodotorula, of which a species of each has been isolated from paper documents,136 the same happens for Eurotium that was detected on Italians documents ancient.68 Gliomastix is a genus that was isolated from the surface of books preserved in a Turkish library.67 It was also isolated from the air of a Cuban museum92 and the atmosphere of Havana.81 The Talaromyces genus has been detected in the air of the Provincial Historical Archive of Santiago de Cuba characterized by having natural ventilation.62 This suggest that the isolates found of Gliomastix and Talaromyces could have penetrated the indoor of the repositories and sedimented on the documents together with the dust, even though they were not detected in the air or in the dust collected from the repositories of the NARC. However, Talaromyces species were isolated from documents preserved at NARC;41,43 and there are references to the species T. wartmannii and T. flavus which were isolated from the of documents preserved in Polish and Greek archives, libraries, and museum.25,64
From the 98 isolated species, two turned out to be abundant (Aspergillus flavus, A. niger), two were classified as common (Penicillium sp., Penicillium citrinum), six were frequent (Candida sp., Chrysogenum sp., Rhizopus sp., Trichoderma sp.), 24 were classified as occasional and 64 were rare (Figure 4B, Table 1). Likewise, 27 species turned out to be from the Aspergillus genus (26.7%), five of Cladosporium (5%), 24 of Penicillium (23.8%) and the remaining percentage corresponded to species of other genera (Figure 5). Aspergillus flavus and A. niger are species that have been detected in other studies,74 while P. citrinum and P. chrysogenum have been commonly isolated from documents and reported by other authors.9,136,149 On the other hand, Aspergillus niger and P. chrysogenum were among the isolated species of Moroccan documents.150
Although Chaetomium globosum, Cryptococcus sp., Rhodotorula sp. and Candida sp., turned out to be rare species in the isolations from documents preserved in the NARC, they have been detected on the document surfaces in paper and other materials conserved in other countries.148,149
Similarity of genera and species detected in the three niches
Table 2 shows the genera that were similarly detected in the three analyzed niches. As can be seen, the same six genera were isolated from air, dust, and the document surfaces (Alternaria, Aspergillus, Chaetomium, Cladosporium, Curvularia, Penicillium), while 19 genera were detected in two of the analyzed niches. From these, 14 (73.7%) were isolated from both air and dust (Candida, Cephalosporium, Fusarium, Gliocladium, Mucor, Paecilomyces, Phoma, Rhizopus, Rhodotorula, Sporotrichum, Trichoderma, Trichothecium, Trichophyton, Verticillium), four genera (21.1%) were detected in air and on document surfaces (Harposporium, Neurospora, Scolecobasidium, Zygosporium) and only one genus (Eurotium) was detected in dust and on document surfaces (5.2%).
|
No. |
Genera |
Air |
Settled dust |
Documents surface |
RF (%) |
EC |
|
1 |
Acremonium |
+ |
- |
- |
33.3 |
O |
|
2 |
Alternaria |
+ |
+ |
+ |
100 |
A |
|
3 |
Aspergillus |
+ |
+ |
+ |
100 |
A |
|
4 |
Aureobasidium |
+ |
- |
- |
33.3 |
O |
|
5 |
Beltraniella |
+ |
- |
- |
33.3 |
O |
|
6 |
Bipolaris |
+ |
- |
- |
33.3 |
O |
|
7 |
Blastomyces |
+ |
- |
- |
33.3 |
O |
|
8 |
Botryoderma |
+ |
- |
- |
33.3 |
O |
|
9 |
Botryotrichum |
+ |
- |
- |
33.3 |
O |
|
10 |
Candida |
+ |
- |
+ |
66.7 |
C |
|
11 |
Cephalosporium |
+ |
- |
+ |
66.7 |
C |
|
12 |
Chaetomium |
+ |
+ |
+ |
100 |
A |
|
13 |
Chrysonilia |
+ |
- |
- |
33.3 |
O |
|
14 |
Chrysosporium |
+ |
- |
- |
33.3 |
O |
|
15 |
Cladosporium |
+ |
+ |
+ |
100 |
A |
|
16 |
Curvularia |
+ |
+ |
+ |
100 |
A |
|
17 |
Cryptococcus |
- |
- |
+ |
33.3 |
O |
|
18 |
Emiricella |
- |
- |
+ |
33.3 |
O |
|
19 |
Eurotium |
- |
+ |
+ |
66.7 |
C |
|
20 |
Epicoccum |
+ |
- |
- |
33.3 |
O |
|
21 |
Exophiala |
+ |
- |
- |
33.3 |
O |
|
22 |
Fusarium |
+ |
- |
+ |
66.7 |
C |
|
23 |
Geotrichum |
+ |
- |
- |
33.3 |
O |
|
24 |
Gilmaniella |
+ |
- |
- |
33.3 |
O |
|
25 |
Gliocladium |
+ |
- |
+ |
66.7 |
C |
|
26 |
Gliomastix |
- |
- |
+ |
33.3 |
O |
|
27 |
Gymnoascus |
- |
- |
+ |
33.3 |
O |
|
28 |
Harposporium |
+ |
+ |
- |
66.7 |
C |
|
29 |
Hormiscium |
+ |
- |
- |
33.3 |
O |
|
30 |
Humicola |
- |
+ |
- |
33.3 |
O |
|
31 |
Hyalodendriella |
+ |
- |
- |
33.3 |
O |
|
32 |
Itersonilia |
+ |
- |
- |
33.3 |
O |
|
33 |
Monilia |
+ |
- |
- |
33.3 |
O |
|
34 |
Mucor |
+ |
- |
+ |
66.7 |
C |
|
35 |
Neurospora |
+ |
+ |
- |
66.7 |
C |
|
36 |
Nigrospora |
+ |
- |
- |
33.3 |
O |
|
37 |
Nodulisporium |
+ |
- |
- |
33.3 |
O |
|
38 |
Ovulariopsis |
+ |
- |
- |
33.3 |
O |
|
39 |
Paecilomyces |
+ |
- |
+ |
66.7 |
C |
|
40 |
Papularia |
+ |
- |
- |
33.3 |
O |
|
41 |
Papulaspora |
+ |
- |
- |
33.3 |
O |
|
42 |
Penicillium |
+ |
+ |
+ |
100 |
A |
|
43 |
Pestalotia |
+ |
- |
- |
33.3 |
O |
|
44 |
Phoma |
+ |
- |
+ |
66.7 |
C |
|
45 |
Rhizopus |
+ |
- |
+ |
66.7 |
C |
|
46 |
Rhodotorula |
+ |
- |
+ |
66.7 |
C |
|
47 |
Scolecobasidium |
+ |
+ |
- |
66.7 |
C |
|
48 |
Scopulariopsis |
+ |
- |
- |
33.3 |
O |
|
49 |
Sepedonium |
+ |
- |
- |
33.3 |
O |
|
50 |
Sprorobolomyces |
+ |
- |
- |
33.3 |
O |
|
51 |
Sporotrichum |
+ |
- |
+ |
66.7 |
C |
|
52 |
Stachybotrys |
- |
- |
+ |
33.3 |
O |
|
53 |
Stemphylium |
+ |
- |
- |
33.3 |
O |
|
54 |
Syncephalastrum |
+ |
- |
- |
33.3 |
O |
|
55 |
Talaromyces |
- |
- |
+ |
33.3 |
O |
|
56 |
Trichoderma |
+ |
- |
+ |
66.7 |
C |
|
57 |
Trichothecium |
+ |
- |
+ |
66.7 |
C |
|
58 |
Trichophyton |
+ |
- |
+ |
66.7 |
C |
|
59 |
Torula |
+ |
- |
- |
33.3 |
O |
|
60 |
Verticillium |
+ |
- |
+ |
66.7 |
C |
|
61 |
Wardomyces |
+ |
- |
- |
33.3 |
O |
|
62 |
Zygosporium |
+ |
+ |
- |
66.7 |
C |
|
|
NSM |
+ |
+ |
+ |
100 |
A |
Table 2 Similarity of genera detected in the studied niches according to all the analyzed reports
NSM, Indicates non-sporulating mycelia. Ecological categories (EC), Abundant (A) with RF = 100 – 81%; Common (C) with RF = 80 – 61%; Frequent (F) with RF = 60 – 41%; Occasional (O) with RF = 40 –21%; Rare (R) with RF = 20 – 0.1%.
Regarding the referenced species, it was observed that 58 were able to grow in at least two of the niches analyzed (29%), while only 19 were found in the three niches (9.5%), which is why they are classified as abundant (Table 3). Although a total of 40 Aspergillus species were discovered, 15 were isolated from at least two of the niches analyzed (37.5%) and of them, six were detected in all three niches (15%). From a total of 24 Cladosporium species that were reported, five were found in at least two niches (20.8%) and three were isolated from all three niches (12.5%). A total of 36 species of Penicillium were reported, from which 11 were found in at least two niches (30.6%) and six were detected in all three niches (16.7%).
|
No. |
Species |
Air |
Settled dust |
Documents surface |
RF (%) |
EC |
|
1 |
Alternaria sp. |
+ |
+ |
+ |
100 |
A |
|
2 |
A. geophila |
+ |
- |
+ |
66.7 |
C |
|
3 |
A. solani |
+ |
- |
+ |
66.7 |
C |
|
4 |
Aspergillus sp. |
+ |
- |
+ |
66.7 |
C |
|
5 |
A. alliaceus |
+ |
- |
+ |
66.7 |
C |
|
6 |
A. caespitosus |
+ |
- |
+ |
66.7 |
C |
|
7 |
A. candidus |
+ |
- |
+ |
66.7 |
C |
|
8 |
A. chevalieri* |
- |
+ |
+ |
66.7 |
C |
|
9 |
A. clavatus |
+ |
+ |
- |
66.7 |
C |
|
10 |
A. flavipes |
+ |
- |
+ |
66.7 |
C |
|
11 |
A. flavus |
+ |
+ |
+ |
100 |
A |
|
12 |
A. fumigatus |
+ |
- |
+ |
66.7 |
C |
|
13 |
A. glaucus |
+ |
+ |
+ |
100 |
A |
|
14 |
A. nidulans |
+ |
- |
+ |
66.7 |
C |
|
15 |
A. niger |
+ |
+ |
+ |
100 |
A |
|
16 |
A. ochraceus |
+ |
- |
+ |
66.7 |
C |
|
17 |
A. ornatus |
+ |
- |
+ |
66.7 |
C |
|
18 |
A. oryzae |
+ |
+ |
+ |
100 |
A |
|
19 |
A. panamensis |
+ |
- |
+ |
66.7 |
C |
|
20 |
A. parasiticus |
+ |
- |
+ |
66.7 |
C |
|
21 |
A. penicillioides |
+ |
+ |
- |
66.7 |
C |
|
22 |
A. restrictus |
+ |
+ |
- |
66.7 |
C |
|
23 |
A. terreus |
+ |
+ |
+ |
100 |
A |
|
24 |
A. versicolor |
+ |
+ |
+ |
100 |
A |
|
25 |
Candida sp. |
+ |
- |
+ |
66.7 |
C |
|
26 |
Cephalosporium sp. |
+ |
- |
+ |
66.7 |
C |
|
27 |
Chaetomium sp. |
+ |
+ |
+ |
100 |
A |
|
28 |
C. globosum |
+ |
+ |
+ |
100 |
A |
|
29 |
Cladosporium sp. |
+ |
- |
+ |
66.7 |
C |
|
30 |
Cl. caryigenum |
+ |
+ |
+ |
100 |
A |
|
31 |
Cl. cladosporioides |
+ |
+ |
+ |
100 |
A |
|
32 |
Cl. elatum |
+ |
+ |
- |
66.7 |
C |
|
33 |
Cl. herbarum |
+ |
+ |
+ |
100 |
A |
|
34 |
Cl. oxysporum |
+ |
+ |
- |
66.7 |
C |
|
35 |
Cl. sphaerospermum |
+ |
+ |
- |
66.7 |
C |
|
36 |
Cl. tenuissimum |
+ |
+ |
- |
66.7 |
C |
|
37 |
Curvularia sp. |
+ |
+ |
+ |
100 |
A |
|
38 |
C. lunata |
+ |
- |
+ |
66.7 |
C |
|
39 |
Fusarium sp. |
+ |
- |
+ |
66.7 |
C |
|
40 |
F. solani |
+ |
- |
+ |
66.7 |
C |
|
41 |
F. oxysporum |
+ |
- |
+ |
66.7 |
C |
|
42 |
Gliocladium sp. |
+ |
- |
+ |
66.7 |
C |
|
43 |
Harposporium sp. |
+ |
+ |
- |
66.7 |
C |
|
44 |
Mucor sp. |
+ |
- |
+ |
66.7 |
C |
|
45 |
M. racemosus |
+ |
- |
+ |
66.7 |
C |
|
46 |
Neurospora sp. |
+ |
+ |
- |
66.7 |
C |
|
47 |
Paecilomyces sp. |
+ |
- |
+ |
66.7 |
C |
|
48 |
P. variotii |
+ |
- |
+ |
66.7 |
C |
|
49 |
Penicillium sp. |
+ |
+ |
+ |
100 |
A |
|
50 |
P. aurantiogriseum |
+ |
+ |
- |
66.7 |
C |
|
51 |
P. chrysogenum |
+ |
+ |
+ |
100 |
A |
|
52 |
P. citrinum |
+ |
+ |
+ |
100 |
A |
|
53 |
P. commune |
+ |
+ |
+ |
100 |
A |
|
54 |
P. decumbens |
+ |
+ |
+ |
100 |
A |
|
55 |
P. fellutanum |
+ |
- |
+ |
66.7 |
C |
|
56 |
P. frequentans |
+ |
- |
+ |
66.7 |
C |
|
57 |
P. funiculosum |
+ |
- |
+ |
66.7 |
C |
|
58 |
P. implicatum |
+ |
- |
+ |
66.7 |
C |
|
59 |
P. janczewskii |
+ |
+ |
+ |
100 |
A |
|
60 |
P. janthinellum |
+ |
- |
+ |
66.7 |
C |
|
61 |
P. purpurogenum |
+ |
- |
+ |
66.7 |
C |
|
62 |
P. simplicissimum |
+ |
+ |
- |
66.7 |
C |
|
63 |
P. terrestre |
+ |
- |
+ |
66.7 |
C |
|
64 |
P. viridicatum |
+ |
- |
+ |
66.7 |
C |
|
65 |
P. waksmanii |
+ |
- |
+ |
66.7 |
C |
|
66 |
Phoma sp. |
+ |
- |
+ |
66.7 |
C |
|
67 |
Rhizopus sp. |
+ |
- |
+ |
66.7 |
C |
|
68 |
R. nigricans |
+ |
- |
+ |
66.7 |
C |
|
69 |
R. oryzae |
+ |
- |
+ |
66.7 |
C |
|
70 |
Rhodotorula sp. |
+ |
- |
+ |
66.7 |
C |
|
71 |
Scolecobasidium sp. |
+ |
+ |
- |
66.7 |
C |
|
72 |
Sporotrichum sp. |
+ |
- |
+ |
66.7 |
C |
|
73 |
Trichoderma sp. |
+ |
- |
+ |
66.7 |
C |
|
74 |
T. lignorum |
+ |
- |
+ |
66.7 |
C |
|
75 |
T. viride |
+ |
- |
+ |
66.7 |
C |
|
76 |
Verticillium sp. |
+ |
- |
+ |
66.7 |
C |
|
77 |
Zygosporium sp. |
+ |
+ |
- |
66.7 |
C |
Table 3 Similarity of species detected in at least two of the three ecological niches studied
*, Indicates that it was also isolated as Eurotium chevalieri. Ecological categories (EC), Abundant (A) with RF = 100 – 81%; Common (C) with RF = 80 – 61%; Frequent (F) with RF = 60 – 41%; Occasional (O) with RF = 40 –21%; Rare (R) with RF = 20 – 0.1%.
Although fungal isolations from dust and the document surfaces are still scarce, it is evident that the greatest species diversity was isolated from the air and that the species similarities among these three niches are low, therefore air is the matrix that contributes with the most fungal species to the NARC environment. This result was expected since most of its repositories have natural cross ventilation and outdoor air is considered by many authors to be the largest source of fungal propagules in indoor environments.74,114,130,151,152
A total of 168 fungal genera and 54 species were isolated from air, 36 genera and 12 species from settled dust as well as 98 genera and 26 species were detected on the document surfaces, with a marked predominance of the genera Aspergillus, Cladosporium and Penicillium in the three niches, although 24 rare genera were detected in air after the year 2000 (e.g. Acremonium, Aureobasidium, Beltraniella, Bipolaris, Blastomyces, Botryoderma, Botryotrichum, Chaetomium, Chrysonilia, Exophiala, Gilmaniella, Harposporium, Hyalodendriella, Itersonilia, Nodulisporium, Ovulariopsis, Papularia, Scolecobasidium, Sepedonium, Sprorobolomyces, Torula, Trichophyton, Wardomyces, Zygosporium). Only six genera were similarly isolated from air, dust and the document surfaces (9.7%), while 19 species were found in these three niches (9.5%) (e.g. A. flavus, A. glaucus, A. niger, A. oryzae, A. terreus, A. versicolor, C. globosum, Cl. caryigenum, Cl. cladosporioides, Cl. herbarum, P. chrysogenum, P. citrinum, P. commune, P. decumbens, P. janczewskii). Although fungal isolations from dust and the document surfaces are still scarce, it is evident that the greatest species diversity was isolated from the air, and that the species similarities among these three niches are low, therefore air is the matrix that contributes with the most fungal species to the NARC environment. This compilation of the fungal diversity in the environment of the National Archive of the Republic of Cuba (NARC) repositories will serve as a reference for future studies in Cuban archives and other countries.153−171
None.
Authors declare that there is no conflict of interest.

©2023 Borrego. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.