Journal of
eISSN: 2373-437X


Review Article Volume 10 Issue 5
1Former Vice President of Al Azhar University, Palestine
2Faculty of Science, Geology Department, Menoufia University, Egypt
Correspondence: Haidar Salim Anan, Former Vice President of Al Azhar University-Gaza, P.O. Box 1126, Palestine, Tel 00970 598 838333
Received: October 12, 2022 | Published: October 31, 2022
Citation: Anan HS, Orabi OH. Four and five-chambered evolution of the late maastrichtian planktic foraminiferal Plummerita species in the Tethys. J Microbiol Exp. 2022;10(5):181-187. DOI: 10.15406/jmen.2022.10.00371
Brönnimann1 proposed two groups from Trinidad, which have spine-like prolongation of ultimate and penultimate chambers of the diagnostic latest Maastrichtian planktonic foraminiferal genus Plummerita. As a separate group of this genus, some of it has the last six-chambered volition in Plummerita reicheli group (P. reicheli, P. pustulata, and P. hexacamerata). The same author also proposed another group related to this genus, with the last five-chambered volution: P. hantkeninoides group (P. hantkeninoides, P. costata and P. inflata). Nonetheless, another third group was also added to these Plummerita assemblages, where Anan2 added a distinct species of Plummerita from the Duwi section, the Red Sea coast of Egypt, P. haggagae, which differs from the other species of the Plummerita by its four-chambered volution in the last whorl. In the current study, an attempt is made to identify three new species of the planktic foraminiferal assemblages of five-chambers volution, P. hantkeninoides group (P. hodae, P. kellerae, P. premolisilvae) is believed here to be new, as well as another one four-chamber volution, P. haggagae group (P. elkefensis, n. sp.), which described from many localities in the Southern Tethys (Tunisia, Egypt, Iraq), and Northern Tethys (Italy). The biostratigraphy, paleogeography, paleoecology, and paleobathymetry of the recorded Plummerita members in different Tethyan localities are presented and discussed.
Keywords: Plummerita, Foraminifera, Maastrichtian
The test of the genus Plummerita is stellate with inflated triangular radially elongated chambers in a low to flat trochospiral and ending in a tubulospine, with surface ornamentation (asteroid or spines with rugosity and costellate in meridional alignment), primary aperture interiomarginal, umbilical covered by tegilla, and meridionally arranged costellae on the surface of the chambers. It is also distinguished by 4-6 chambers in the final whorl (Brönnimann1, Loeblich and Tappan)3. Brönnimann1 distinguished, from Trinidad, three subspecies of the last five-chambered volution of Plummerita hantkeninoides (hantkeninoides, costata, inflata) based on the surface ornamentation (asteroid or spines), recorded within the Late Maastrichtian Globotruncana mayaroensis Zone. Plummerita hantkeninoides has highly characteristic by having radially elongated chambers possessing axially situated spines in the final whorl. These spines may exist in some or all chambers of the last whorl, and the chambers that lack spines are triangular and inflated. Masters4 considered the latest Maastrichtian three subspecies of P. hantkeninoides to represent ontogenetic stages of a single adult species P. reicheli and occur after the extinction of A. mayaroensis. This opinion is not accepted by Anan5 and this study, because Brönnimann1 himself distinguished the last six-chambered volition (P. reicheli, pustulata, hexacamerata) as a separate group from another last five-chambered volition P. hantkeninoides group (hantkeninoides, costata, inflata). Moreover, Anan6 (pl. 1, figures5, 6) added another distinct species of Plummerita from the Duwi section, Red Sea coast of Egypt (Figure 1), P. haggagae, which differs from the other species of the Plummerita by its four-chambered volution in the last whorl with a strongly rugose surface, low trochoidal volution, wide and deep umbilical area, radiate ridges and axially pointed spine-like prolongation for the three penultimate chambers with axially situated spines, while the end chamber is inflated in a radial chamber without possessing a spine. Another three new species of the five-chambers P. hantkeninoides group (P. hodae, P. kellerae, P. premolisilvae) and also one new four-chambers P. haggagae group (P. elkefensis) are believed here to be new.

Figure 1 Location map of Duwi section, Red Sea coast, Egypt in the Southern Tethys.65
Nakkady7 recorded Rugoglobigerina reicheli from the Late Maastrichtian rocks of the Duwi section, Red Sea coast (represents one of the classic localities of the Late Cretaceous/ Paleogene succession in Egypt), as well as Tethys and Atlantic basins. Kerdany & Abdelsalam8 recorded P. hantkeninoides and R. reicheli hexacamerata among a rich Maastrichtian foraminiferal assemblage overlying the Gansserina gansseri Zone in the Duwi section and erected a new Maastrichtian species Globotruncana falsocalcarata with these taxa. In Tunisia, another new species Globotruncanella kefennsoura was erected by Solakius9 from the Late Maastrichtian A. mayaroensis and G. falsocalcarata Zones, but without Plummerita spp. in the assemblage. Masters10 concluded that A. mayaroensis becomes extinct before the end of the Maastrichtian, and his P. reicheli Zone occurs in the Esna Shale of Egypt as it has done in El Haria Shale of Tunisia, above the extinction of A. mayaroensis. Thus, where the Maastrichtian is most complete, the A. mayaroensis Zone does not represent the latest Cretaceous and the above interval contains Plummerita reicheli and occasionally Globotruncana falsocalcarata.
The P. hantkeninoides Zone represents the latest Maastrichtian biozone in many localities in the Tethys (Figure 2): Trinidad,11 Mexico,12 Spain,13 Italy,14 Romania,15 Bulgaria,16 Tunisia,17 Egypt,18 Keller,19 Samir,20 Galal,21 Anan,6 Obaidalla et al.,22 Iraq,23 Iran,24 Gallala25 concluded that El Kef section (K/Pg boundary stratotype section and GSSP point) and Ellès section in Tunisia, Agost and Caravaca sections (Betic Cordillera, Spain), relative to the Tethyan realm (low latitude), and Bidart section (France) relative to the Atlantic realm (middle latitude) are complete sections containing all the zones and subzones characterizing the upper Maastrichtian-lower Paleogene interval without any hiatus.

Figure 2 The paleogeographic map of terminal cretaceous showing some studied locations in the open sea Tethys (onlinelibrary wiley.com).
The stratigraphic range (thickness in m.) of the P. hantkeninoides Zone in Spain reaches about 3.5 m in the type locality of P. hantkeninoides Zone in Spain (Pardo et al.13), but 6-9 m in Tunisia,26 1-2 m in some localities in Egypt,27 Keller,19 Galal,28Anan;5,6 but 1.4 m in Italy.12
The asteroid, rugose, costellate in meridional alignment and axially last four and five-chambered volution spine-like prolongation assemblage Plummerita hantkeninoides, P. costata, P. inflata are illustrated (Figure 3. A-I). Another three five-chambered volutions and one four-chambered volution new species are believed to be new: P. hodae, P. kellerae, and P. premolisilvae (Figure 4. A-C) and P. elkefensis (Fig. 4. D, E), respectively. These Plummerita spp. have been reported, described, and illustrated in some Tethyan localities with some added comments by the present authors. The classification of Loeblich & Tappan3 is followed here.
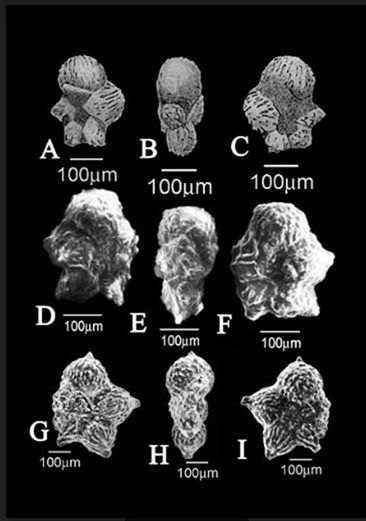
Figure 3 Scale bar = 100 µm
Nos. A-C, Plummerita inflata, (A. Ventral view, B. Side view, C. Dorsal view), the figured holotype;1 Nos.D-F, Plummerita costata (D. Ventral view, E. Side view, F. Dorsal view), the figured holotype;1 Nos. G-I, Plummerita hantkeninoides, G. Ventral view, H. Side view, I. Dorsal view, the figured holotype.1
Order Foraminiferida Eichwald, 1830
Suborder Globigerinina Delage & Hérouard, 1896
Superfamily Globotruncanacea Brotzen, 1942
Family Rugoglobigerinidae Subbotina, 1959
Genus Plummerita Brönnimann, 1952
Type species: Rugoglobigerina (Plummerella) hantkeninoides subspecies hantkeninoides Brönnimann, 1952
Plummerita inflata (Brönnimann, 1952) - (Figure 3. A-C)
Rugoglobigerina (Plummerella) hantkeninoides inflata Brönnimann1, p. 40, pl. 3, figs. 7-9, text-fig. 19a-m.
Plummerita sp. Kassab,29 p. 350, pl. 2, fig. 5.
Plummerita reicheli (Brönnimann) – Masters,4 p. 267, pl. 2, figs. 1, 3, 4.
Plummerita hantkeninoides (Brönnimann) – Salaj,30 p. 302, pl. 16, fig. 12 - Ismail31, p. 332, pl. 3, fig. 10 – Keller,19 p. 81, pl. 2, fig. 10 (non figs. 7-9) – Obaidalla,32 p. 214, pl. 1, fig. 8.
Plummerita inflata (Brönnimann). Anan,5 p. 594, pl. 1, fig. 3.
Remarks: The first three chambers in the last whorl of P. inflata are characterized by axially pointed spine-like prolongations, while the last two chambers are strongly inflated shapes without spines.
Plummerita costata (Brönnimann, 1952) - (Figure 3. D-F)
Rugoglobigerina (Plummerella) hantkeninoides costata Brönnimann,1 p. 40, pl. 3, figs. 4-6, text-fig. 18a-c.
Plummerita reicheli (Brönnimann) – Masters,4 p. 267, pl. 1, figs. 7, 8, pl. 2, fig. 2 - Lüning et al,33 p. 158, fig. 3. 3.
Plummerita hantkeninoides Brönnimann. Keller,19 p. 81, pl. 2, fig. 9 (non figs. 7, 8, 10) - El-Dawy,34 p. 51, pl. 1, figs. 10, 11 - Darvishzad et al,24 p. 142, pl. 2, fig. 1 – Gallala,25 p. 13, fig. 13. 5 (non. 1-4) - Orabi & Zahran,35 p. 80, fig. 1. 10 - Coccioni & Premoli Silva,14 p. 59, pl. 2, figs. 13, 14 (non figs. 12, 15, 16).
Plummerita costata (Brönnimann) – Anan,5 p. 594, pl. 1, fig. 2.
Remarks: P. costata is distinguished by its five-chambered volition in dextral coiling, with axially pointed spine-like prolongation for the four penultimate chambers, but without spine-like prolongation of the last inflated chamber.
Plummerita hantkeninoides (Brönnimann, 1952 – ((Figure 3. G-I)
Rugoglobigerina (Plummerella) h. hantkeninoides Brönnimann,1 p. 37, pl. 3, figs. 1-3, text-fig.1a-k - Bolli et al,11 p. 43, pl. 43, fig. 5.
Plummerita hantkeninoides (Brönnimann) - Almogi-Labin et al,36 p. 48, pl. 1, figs. 8, 9.
Plummerita reicheli (Brönnimann) – Masters,4 p. 267, pl. 1, figs. 1-6, pl. 3, fig. 1- Luger27, p. 46, pl. 2, figs. 4,9,14 - Robaszynski et al.37, p. 476. pl. 18, fig. 5.
Plummerita hantkeninoides (Brönnimann). Arz et al,38 p. 224, pl.1, fig. 7- Karoui-Yaakoub et al,39 p. 241, pl. 1, fig. 5 – Anan,40 p. 300, pl. 1, fig. 5 - Coccioni & Premoli Silva,14 p. 59, pl. 2, fig. 12 (non figs. 13-16).
Remarks: P. hantkeninoides is characterized by the last five-chambered volition, radially elongated with axially spine-like prolongation of linear pattern rugose surface for all the five chambers in the last whorl.
Plummerita hodae Anan, n. sp. - (Fig. 4. A)
Plummerita inflata (Brönnimann). Anan,5 p. 594, pl. 1, fig. 3.
Holotype: Fig. 4. A
Dimension: Scale bar=100 µm.
Depository: Geology Department, Ain Shams University-Cairo, Anan collection (ASUGD A38).
Etymology: In the memory of the late mother of the first author Hoda Anan.
Type level and locality: It is recorded with the latest Maastrichtian Plummerita hantkeninoides Zone, Duwi section, Red Sea coast, Egypt.
Description: It is distinguished by its radially elongated chambers with axially spine-like prolongation in linear pattern rugose surface in the last whorl with low trochoidal volution. The first three chambers of P. hodae have axially protruding pointed spine-like prolongations. The last two chambers are strongly inflated in shape, but without spines for the last fourth and fifth chambers in the last whorl.
Discussion: Plummerita hodae n. sp. has the last five chambered volutions as P. hantkeninoides, P. costata, and P. inflata Brönnimann.1 It is closely related to the latter species P. inflata (Figure 3.1) but differs from it by the position of its larger fourth chamber than the last fifth chamber, and the third chamber exists perpendicular along the vertical line to the last fifth chamber of the test in the final whorl of the ventral side.
Plummerita kellerae Anan & Orabi, n. sp. - (Figure 4. B)
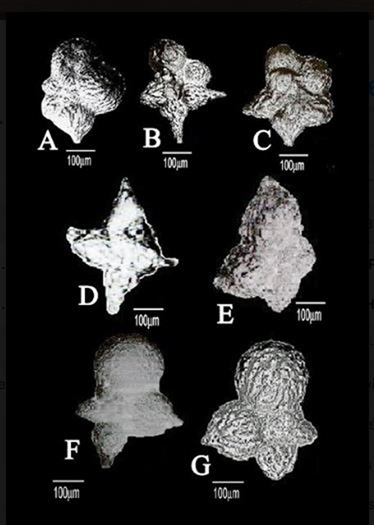
Figure 4 Scale bar = 100 µm.
A. Plummerita hodae Anan, n. sp., (Dorsal view), the illustrated holotype from Duwi section,Egypt.5
B. Plummerita kellerae Anan and Orabi, n. sp., (Ventral view), the illustrated holotype from El Kef section, Tunisia.19
C. Plummerita isabellae Anan and Orabi, n. sp. (Dorsal view), the illustrated holotype from Gubbio section, Italy.14
D, E. Plummerita elkefensis Anan and Orabi, n. sp., D. (Dorsal view), the figured hypotype,20 E. (Dorsal view), the figured holotype.17
F, G. Plummerita haggagae Anan2, F. (Dorsal view), the figured hypotype,18 G. (Dorsal view), the figured holotype.2
Plummerita hantkeninoides Keller et al,19 p. 279, pl. 2, fig. 14.
Holotype: Fig. 4. B.
Dimension: Scale bar=100 µm.
Depositary: The collection of the American paleontologist G. Keller.
Etymology: In the honor of the American paleontologist Gerta Keller, Geosciences Department, Princeton University, Princeton, NJ 08544, USA.
Type locality and level: The top 50 cm below the K/T boundary, El Kef section, Tunisia.
Age: Latest Maastrichtian Plummerita hantkeninoides Zone.
Description: It is distinguished by its first three radially elongated chambers with axially spine-like prolongation in linear pattern rugose surface in the last whorl with low trochoidal volution, while the fourth and fifth-last chambers are strongly inflated without spine-like prolongation. The third chamber with axially spine-like prolongation of P. kellerae exists perpendicular to the last fifth chamber at the vertical line of the test.
Discussion: Plummerita kellerae n. sp. (Figure 4. B) has closely related to the other species P. inflata (Figure 3. A-C) and P. hodae (Figure 4. A), but differs from it by its first three radially elongate chambers with axially spine-like prolongation, while the last penultimate and ultimate chambers are inflated without spines.
Plummerita premolisilvae Anan & Orabi, n. sp. - (Figure 4.C)
Plummerita hantkeninoides (Brönnimann). Coccioni & Premoli Silva,14 p. 59, pl. 3, fig. 15 (non figs. 12-14, 16).
Holotype: Fig. 4. C.
Dimension: Scale bar=100 µm.
Depository: Sample Bottaccione section, BTT 2067 (381.2 m), Gubbio, Italy.
Etymology: In the honor of Italian micropaleontologist Isabella Premoli Silva, Dipartimento di Scienze Della Terra, Ardito Desio, Universita degli Studi di Milano, Italy.
Age: Latest Maastrichtian Plummerita hantkeninoides Zone.
Description: It is distinguished by its first two radially elongated chambers with axially spine-like prolongation in linear pattern rugose surface in the last whorl with low trochoidal volution, while the third, fourth, and fifth-last chambers are strongly inflated without spine-like prolongation. The second chamber with axially spine-like prolongation of P. premolisilvae exists perpendicular to the last fifth chamber at the vertical line of the test.
Discussion: Plummerita premolisilvae n. sp. (Figure 4. C) has also closely related to the other species P. inflata (Figure 3. A-C) and P. kellerae (Figure 4. B), but differs from it by its only first two radially elongate chambers with axially spine-like prolongation, while the last third, fourth and fifth last chambers are inflated without spines.
Plummerita elkefensis Anan and Orabi, n. sp. - (Figure 4. D, E)
Plummerita hantkeninoides (Brönnimann). Samir,20 p. 24, pl. 1, fig. 5.
Plummerita hantkeninoides (Brönnimann). Keller,17 p. 741, pl. 16.4.
Holotype: Fig. 4. D, E
Dimension: Scale bar = 100 µm.
Depositary: The American paleontologist G. Keller collection.
Etymology: After El Kef section, Tunisia.
Age: Latest Maastrichtian Plummerita hantkeninoides Zone.
Description: P. elkefensis is characterized by the last four-chambered volition, radially elongated with axially spine-like prolongation of linear pattern rugose surface for all the four chambers in the last whorl.
Discussion: It is distinguished by its radially elongated chambers with axially spine-like prolongation in linear pattern rugose surface in all four chambers in the last whorl with low trochoidal volution.
Plummerita haggagae Anan, 2008 - (Figure 4. F, G)
Plummerita hantkeninoides (Brönnimann) - Ziko et al,18 p. 143, fig. 4. 10, 11 – Keller,19 p. 81, fig. 8 (non-figs. 9, 10) – Galal,28 p. 246, fig. 7.4 - Obaidalla et al,22 p. 67, fig. 18.K (non-J) - Bamerni et al.,41 p. 8, pl. 1, figs. 5-8.
Plummerita haggagae Anan,6 p. 249, pl. 1, figs. 2,3 – Anan,5 p. 594, pl. 1, figs. 5, 6.
Description: P. haggagae is distinguished by its four-chambered volition in dextral coiling, with axially pointed spine-like prolongation for the three penultimate chambers, but without spine-like prolongation of the last fourth inflated chamber. It was recorded, so far, from Egypt and Iraq.
Keller42 worked on the high-latitude K/P transition deposits (sites 738C, 752B, 690C) and noted the absence of P. hantkeninoides. Consequently, if these species were considered absent in the middle- and high-latitude areas, they would be restricted to low latitudes Keller et al.,43 used this small-sized species (<150 mm) as the biomarker of the latest Maastrichtian nominate zone. Keller19 noted that among Rugoglobigerinids, only P. hantkeninoides, Trinitella scotti, and possibly Rugoglobigerina reicheli are largely restricted to the low latitude Tethys, and these surface dwellers rarely appear in middle latitudes and are generally absent beyond 30° north or south. Gallala25 noted that P. hantkeninoides spp. is absent at middle latitudes: Bidart section (SW France) and Zumaya section (Spain).
One or more representative species of the genus Plummerita hantkeninoides group were firstly recorded from Trinidad (Caribbean Sea) by Brönnimann,1 and after on, it was recorded partly or completely from many other localities in the Tethys, i.e. Colombia,44 Brazil,45 Trinidad,1,11 Mexico,38 Spain,13 Italy,14 Romania,15 Bulgaria,16 Tunisia,46,39 Egypt,8,10 Jordan,47 Iraq,29,23 Iran,24 Pakistan,48 Indian Ocean,49 (Figures 5,6). According to available kinds of literature, it does not record from other Tethyan localities, which may be due to a lack of studies in planktic foraminifera, hiatus around the Cretaceous/Tertiary (K/T) boundary, and/or opportunist climatic conditions. The representative species of the P. haggagae were originally recorded from Egypt,6,17,18,20-22 while P. elkefensis were recorded from Egypt,20 and Tunisia.17
Frerichs50 noted that the radiations of planktonic foraminiferal genera are characteristic of the warm stratigraphic intervals, and the oxygenic level of the atmosphere should be low during times of extinction (e.g. K/T boundary). Dorreen48 noted that the absence of Late Maastrichtian Abathomphalus mayaroensis from the Gaj section in southern Pakistan might well reflect very warm water, while the G. falsocalcarata Zone represents the topmost Maastrichtian.
Smith51 regarded that nearly all species of Late Maastrichtian A. mayaroensis Zone are known from deep water of tropical regions, while G. falsocalcarata, of probable shallow water species, have not been found with the assemblage, while Anan52 recognized the top Maastrichtian A. mayaroensis Zone in Jiran El Ful section, Abu Rawash (west Cairo) with a gradual decrease of planktic foraminifera and P/B ratio toward top Maastrichtian, which indicate cold water. Anan & Hewaidy53 considered the fauna in the Duwi section to be related to the ‘Midway-Type Fauna’ of Berggren,54 a middle-outer neritic environment (50-200 m). Keller55 noted that the absence of A. mayaroensis in El Kef, Tunisia represents shallow water conditions, rather than a hiatus.
Anan & Sharabi56 noted that the upper Maastrichtian dark shales in Kharga Oasis of Egypt containing elongated and rounded red ferruginous mudstone nodules are mainly devoid of index planktonic foraminifera with scarce arenaceous benthonic individuals, which may express shallow environmental conditions, reduced salinities and lowered oxygen levels. Speijer57 noted that the Late Maastrichtian assemblage of Gabal Duwi has very few taxa in common with the bathyal assemblage. Li & Keller58 noted that in the Maastrichtian, a short-lived global warming pulse was recorded in the oceans at 65.78–65.57 Ma.
Speijer et al.,59 noted that the Wadi Nukhul section (Sinai) represents deep basinal deposition (500-600 m), while Gabal Duwi (central Egypt) represents the middle shelf (150-200 m). Alegret et al.,60 interpreted the bathyal depths during the A. mayaroensis through the early P. hantkeninoides Biochrons at the Agost section in southeastern Spain. Anan6 considered Sinai and central Egypt to have a middle-outer neritic environment (50-200 m). Keller19 noted that P. hantkeninoides (in Gabal Qreiya in central Egypt, west of Gabal Duwi) evolved near the lower part of the latest Maastrichtian warm event (~65.3 Ma) and disappeared at the K/T boundary. Keller17 also added that the global sea level low stand at 65.5 Ma is associated with widespread erosion. Al-Wosabi & Abu Shama61 noted that P. hantkeninoides is attractive to warm-water conditions, and El-Sabbagh62 also noted that the peak abundance in P. hantkeninoides Zone in western Sinai may reflect the global warming event between 65.4-65.2 Ma.
Gertsch et al.,63 noted that during the latest Maastrichtian, periodic acid rains (carbonate dissolution; CIA index: 70–80) associated with pulsed Deccan eruptions in India and strong continental weathering resulted in mesotrophic waters, and resulting super-stressed environmental conditions led to the demise of nearly all planktic foraminiferal species and blooms of the disaster opportunist Guembelitria cretacea. Keller64 noted that the discovery of the direct link between Deccan volcanism (west India) and the end-Cretaceous mass extinction also links volcanism to the late Maastrichtian rapid global warming and high environmental stress (Figure 7).
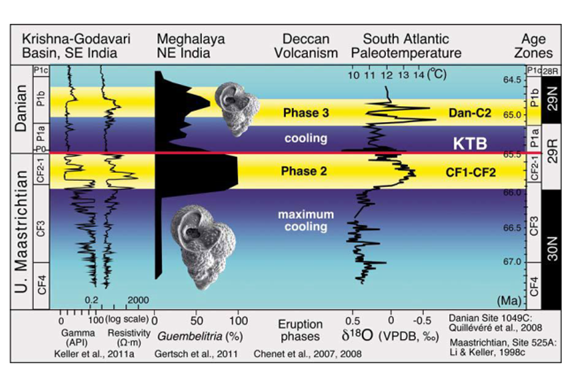
Figure 7 Deccan phase 2 and phase 3 volcanism is correlative with global warming events in the late Maastrichtian zones CF1-CF2 and the early Danian. Dan-C2 event in zone P1b. KTB-Cretaceous-Tertiary boundary; API-American Petroleum Institute units; VPDB-Vienna Peedee belemnite.67
The rich and well-preserved Late Maastrichtian planktic foraminifera Plummerita spp. of the most studied section in the Tethys made it possible to improve the biostratigraphic resolution and to clarify some aspects of the paleoecology, paleoenvironments, and paleogeography. Combining this with data available from previous studies, the following conclusions are presented:
Gratitude was expressed to the editor of JMEN, the unknown reviewers for their valuable comments, and also to my daughter Dr. Huda H. Anan for her help in the development of the figures. First and foremost, we want to sincerely thank Dr. Friedrich W. Van der Wart, an independent researcher in, the Netherlands, for his help during the whole process of this work.
The authors declare that there is no conflict of interest.

©2022 Anan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.