Journal of
eISSN: 2373-437X


Research Article Volume 10 Issue 6
1Colegio de Postgraduados Campus Puebla, Mexico
2Laboratorio de Ecología Molecular Microbiana del CICMICBUAP, Benemérita Universidad Autónoma de Puebla, Mexico
Correspondence: Alfonso Benítez de la Torre, Colegio de Postgraduados Campus Puebla, Km. 12.5 Carretera Federal México-Puebla (s/n) C. P. 72760 Puebla, Mexico, Tel +522222727618
Received: October 26, 2022 | Published: November 8, 2022
Citation: de la Torre AB, Pérez-Ramírez E, Morales-García YE, et al. Evaluation of phytotoxicity of sweet cheese whey in alfalfa and corn. J Microbiol Exp. 2022;10(6):189-193. DOI: 10.15406/jmen.2022.10.00372
The use of cheese whey in agricultural applications is an option to reduce the environmental impact caused when it is dumped without control. The purpose of this work was to measure the phytotoxicity of the sweet whey that is generated during the production of fresh cheese using seeds and seedlings of alfalfa (Medicago sativa L.) and corn (Zea mays L.) as test-object organisms. The variables evaluated were the lethal concentration (LC50) due to the inhibition of germination in both seeds, and the sublethal concentration (CSL) due to the decrease in the development of sprouts and seedlings. Four individual experiments were carried out under controlled laboratory conditions, both in Petri dishes with culture medium and in germinators with support, in a completely randomized experimental design (DECA) with five treatments (4, 8, 12, 16 and 20% of whey and a drinking water control) with a total of 252 experimental units. The results obtained indicate that sweet whey has an LC50-5 of 53% and a CSL-10 of 12% in alfalfa seeds (p<0.05) and an LC50-60 of 20% in alfalfa seedlings and an LC50-20 of 20% in corn seedlings. It was observed at 60 days that at concentrations between 4 and 6%, sweet whey has a growth-promoting effect on corn seedlings (p<0.05), concluding that it is possible to safely use diluted whey in sustainable agricultural practices.
Keywords: bioassay, cheese whey, pollution, use of agro-industrial residues
Whey or cheese whey is the residual liquid from the transformation of milk into cheese, casein and derivatives; it is a translucent liquid obtained by separating the casein coagulum during cheese making.1 Depending on the method used to make cheese, from 100 L of processed milk, between 85 and 90 L of whey are obtained, with retention of up to 55% of the milk's nutrients.2,3 Globally, between 180-190 million tons of whey are generated each year.4,5 It is estimated that in Mexico, artisanal cheese factories generate more than 2.4 million tons per year, but only 50% is processed and transformed into various co-products 6 the rest is discarded in rivers, lakes or on the ground causing serious environmental damage.7 González8 reports a Biological Oxygen Demand (BOD) of 40g/L and a Chemical Oxygen Demand (COD) of 60g/L with a biodegradability index (BOD5/COD) between 0.4 to 0.8 that exceed the limits allowed by the Official Mexican Standard NOM-001-ECOL-1996 on the maximum limits of pollutants in wastewater discharges for agricultural use, which is 0.2 g/L (OGF, 1997). Ramírez9 equates the polluting force of five liters of whey to that of black water produced in one day by one person. Considering its content in lactose, protein and microelements,10,11 various studies have been carried out to take advantage of whey in human and animal nutrition, in the production of bioplastics and fertilizers (Quille30 and for agricultural irrigation.12,13,14 In the Mexican context, where low-tech artisan dairies predominate,11,40 the use of sweet whey in agricultural applications represents a good option, in addition to contributing to the sustainable production and circular economy of milk by reducing contamination due to poor disposal. In this regard, some studies show that when it is poured into agricultural soil it physically and chemically affects its structure15 and consequently, agricultural yield decreases.14,16 However, in other studies it has been shown that most of the solids present in whey are biodegradable, in addition to the fact that its content in Phosphorus, Calcium, Sodium, Potassium, Magnesium and Chloride contributes to improving soil aggregation,17 and its fertility,18,19 recommending its use in agricultural irrigation under specific conditions and only in a controlled manner in dilutions not greater than 20% for obtain an acceptable quality of irrigation water with a maximum of 40 to 60 kg/ha of total salt and with the advantage to integrate nutrients from agro-industrial residues, reducing production costs and reducing the environmental impact of poor disposal of the same.20
Bioassays are tests designed to complement the information provided by traditional chemical tests such as chemical oxygen demand (COD) and biological oxygen demand (BOD) used to assess the damage that a discharge can cause. Its objective is to estimate the toxicity or potential biological impact of exposure to industrial effluents on the physiological function of living organisms.21 In these tests, the concentration that inhibits the germination of 50% of the exposed seeds is determined, which is known as the median lethal concentration (LC50), as well as the concentration at which a sublethal effect (CSL) occurs, characterized by the growth retardation of the seedling (Uc-Peraza and Delgado-Blas, 2012). In addition to the in vitro tests,22 propose phytotoxicity bioassays in hydroponic systems with substrate to evaluate effluents that possibly have a negative effect on root aeration, thus expanding the possibility of knowing the phytotoxic potential of various effluents and their effect on plant species of commercial interest. The purpose of the present work was to demonstrate that it is possible to safely take advantage of the sweet whey that is generated during the production of fresh cheese in the irrigation of alfalfa (Medicago sativa L.) and corn. (Zea mays L.) as test-objet organisms, that is, those that are used for experimentation and in which it is proposed to apply in real conditions, through acute toxicity (LC50) and sublethal effect (CSL) bioassays in the first stages of its development.
The experiments were carried out in the Microbial Molecular Ecology Laboratory of the Center for Research in Microbiological Sciences (CICM) of the Meritorious Autonomous University of Puebla, México (ICUAP-BUAP).
Preparation and analysis of whey: Sweet cheese whey was obtained from the production of fresh cheese at the dairy "Lacteos Galeazzi" in Chipilo Puebla, which is characterized by its low acidity, neutral pH and absence of salts. It was immediately refrigerated at 4°C in 1 L Schott flasks until use. To characterize it, its pH was measured using a Hanna 2010 potentiometer, and its acidity by titration expressed in Dornic degrees (°D) (AOAC, 1995) making determinations in triplicate.
Biological material: Non-sterilized seeds of alfalfa (Medicago sativa L.) and maize (Zea mays L.) selected according to their shape and size, stored in hermetic amber bottles at room temperature, were used.
Experimental design, treatments and variables: A completely randomized experimental design (DECA) was used, evaluating six concentrations of whey in a range between 0 and 100% to measure the variable CL50-5, (concentration of the effluent that causes 50% mortality in the population in 5 days) and six concentrations between 0 and 20% to evaluate the CSL (significant decrease in the development of sprouts and seedlings measured by total biomass, total length and hypocotyl length) in 4 acute and chronic toxicity bioassays (APHA, 1992) with 3-10 repetitions with two plant species in two phenological stages as described in table 1.
|
Variable |
Time (days) |
Species |
Phenological stage |
Experimentation level |
Treatments (chees whey concentration %) |
Repetitions/tratament |
Experimental units |
|
CL50 |
5 |
Alfalfa |
Seed germination |
in vitro* |
0, 20, 40, 60, 80 ,100 |
3 |
18 |
|
CL50 |
5 |
Corn |
Seed germination |
in vitro |
0, 20, 40, 60, 80 ,100 |
3 |
18 |
|
CSL |
10 |
Alfalfa |
Germinated development |
in vitro |
0, 4, 8, 12, 16, 20 |
3 |
18 |
|
CSL |
10 |
Corn |
Germinated development |
in vitro |
0, 4, 8, 12, 16, 20 |
3 |
18 |
|
CL50 |
60 |
Alfalfa |
Seedling emergency |
hydroponic** |
0, 20, 40, 60, 80 ,100 |
5 |
30 |
|
CL50 |
20 |
Corn |
Seedling emergency |
hydroponic |
0, 20, 40, 60, 80 ,100 |
10 |
60 |
|
CSL |
60 |
Alfalfa |
Seedling development |
hydroponic |
0, 4, 8, 12, 16, 20 |
5 |
30 |
|
CSL |
20 |
Corn |
Seedling development |
hydroponic |
0, 4, 8, 12, 16, 20 |
10 |
60 |
|
|
|
|
total |
252 |
|||
Table 1 Experimental design
*In petri dishes, darkness and controlled temperature
**In germinators and falcon tubes sterile vermiculite and controlled temperature-photoperiod conditions
Description of the experiment: To calculate the LC50 of whey in alfalfa and corn seeds, Petri dishes were prepared with Whatman No. 3 paper saturated with 5 mL of each dilution, placing 10 seeds per box and per dilution, separating them as much as possible to allow their development (three boxes per dilution). Each box was sealed with Parafilm®, wrapped in black plastic and incubated at 20±2°C for 5 days.23 The number of germinated seeds was counted by plotting their percentage against the concentration of whey, and the value that produces inhibition of germination in 50% of the population was obtained by extrapolation thus using the equation obtained from polynomial regression. Dead seeds were considered those that did not germinate after 20 days even irrigated with drinking water. The experiment was done three times, with whey from different batches and on different dates, and the results of the most illustrative experiment were reported. The CSL of sweet whey was determined at 10 days using dilutions less than 20%, evaluating the decrease in germinated development (hypocotyl length, length and total weight), under the same conditions of the experiment, according to the method described by Tiquia and Tam.24 To evaluate the LC50 of sweet whey in alfalfa and corn seedlings, a styrofoam germinator was prepared with sterile vermiculite as substrate, sowing 10 alfalfa seeds per well, assigning five wells for each treatment. At the same time, 50 mL Falcon tubes were prepared with 30 mL of sterile vermiculite and one corn seed was sown per tube with 10 replications per treatment. The material was placed under controlled incubation conditions (20±4°C with photoperiod 12-12) and irrigated with 5 mL of each treatment for 20 days in the case of corn with a serological pipette and 60 days for alfalfa with a hypodermic syringe. The number of emerged seedlings was counted, their percentage was plotted against whey concentration, and the value that inhibited emergence in 50% of the population was obtained by interpolation and by means of the equation obtained by regression of the polynomial curve. To determine the CSL of sweet whey in alfalfa and corn seedlings were tested at dilutions less than 20%, evaluating the decrease in development (biomass expressed in dry weight, total length and root length with respect to the drinking water control) according to the method described by Tiquia and Tam24 at 20 days for maize and 60 for alfalfa, starting irrigation with the treatments once the seedlings emerged and watering them at their base. It was considered that there was a significant sublethal effect when the comparison of means between each treatment against the control was lower in a statistically significant way according to the t-Student test (p<0.05).
Statistic analysis: To ensure that the coefficient of variation remained below 30%, a pair of data (maximum and minimum) was eliminated, when necessary. Statistical packages Excel and SAS v 9.4 (2004) were used.
Physicochemical analysis: The results of the laboratory tests performed on the sweet whey generated in Chipilo Puebla were similar to those reported by Panesar25 for sweet whey: pH close to neutrality (6.8 ±0.3) and low acidity (19±0.5 °D). It is important to consider that the characteristics of whey vary depending on the type of cheese that is processed, and that its agricultural use will depend mainly on its being free of added salt. On the other hand, whey, although it has a low acidity at the time it is generated, tends to quickly acidify naturally due to the development of its microbial flora: the lactic acid generated in said process can have a beneficial effect on processes agricultural as in the chelation and solubilization of phosphates26 but it will be a priority to take care of the development of pathogenic microorganisms with controlled fermentation processes, for example with the technology of beneficial and/or efficient microorganisms.27
LC50 in seed germination: As can be seen in figure 1, the sweet whey presented an LC50-5 of 53% in alfalfa seeds, a value that was obtained graphically by interpolation and that coincides by substituting the value y=50 (50% of the population that does not survive) in the polynomial equation. The LC50 in corn was not obtained, because in all the treatments there was contamination by filamentous fungi. This may be due to the natural microbial load of corn, which is favored by the nutrient contribution of whey.
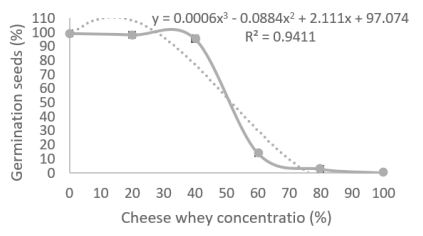
Figure 1 Toxic effect of sweet whey at different concentrations on alfalfa seed germination at 5 days. Corrected values considering that the control presented 83.5% germination.
CSL in the development of sprouts: Sweet whey had a sublethal effect at concentrations greater than 12% on the development of alfalfa sprouts after 10 days of exposure, with significant differences being observed between the total length of sprouts and hypocotyls compared to the drinking water control (p <0.05) (Figure 2), although no effects were observed on total biomass (Figure 3). CSL values for corn were not obtained because, as in the previous experiment, the seeds were contaminated with filamentous fungi in all treatments.
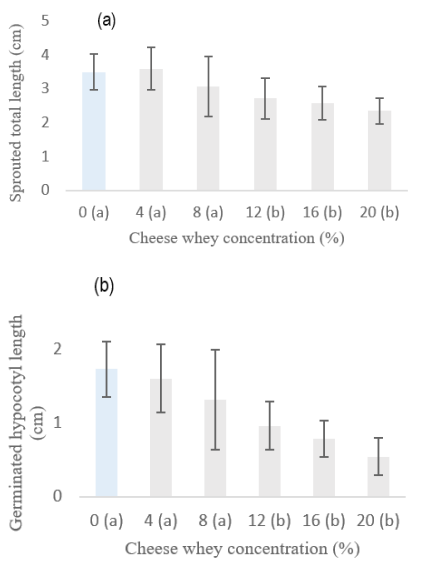
Figure 2 Sublethal effect of sweet whey on total length (a) and hypocotyl length (b) of 10-day-old alfalfa sprouts. Different letters between columns indicate a significant difference (t-Student p<0.05).
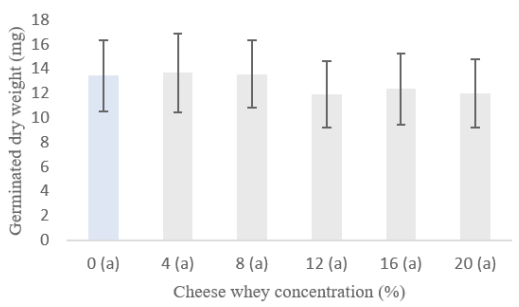
Figure 3 Sublethal effect of sweet whey on the total biomass of 10-day alfalfa sprouts. Equal letters between columns indicate that there is no significant difference (t-Student p<0.05).
LC50 at seedling emergence: The lethal concentration of sweet whey in corn seedlings was obtained at a concentration of 40%, and similarly in alfalfa seedlings; Although 100% lethality was not observed in alfalfa at high whey concentrations, the survival of 50% of the population is evidence that this species is more tolerant to sweet whey than corn (Figure 4).
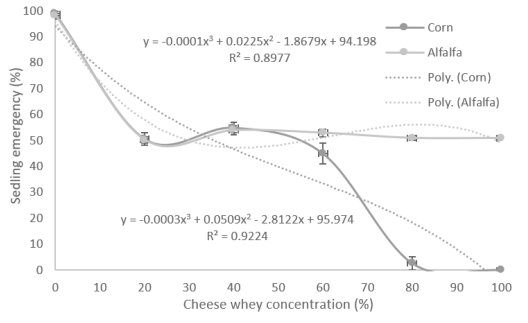
Figure 4 Toxic effect of sweet whey at different concentrations on the emergence of corn (Zea mayz L.) and alfalfa (Medicago sativa L.) seedlings.
CSL in seedling development: Sweet whey did not present a sublethal effect at a concentration of less than 10%, neither in corn seedlings (Figure 5) nor in alfalfa (Figure 6), with a beneficial effect being observed at a concentration of 5±1% for corn (Figure 5a), and between 5±3 % for alfalfa (p<0.05) as shown in figure 6a.
The results presented show the importance of using whey without salt and with a pH close to neutrality for irrigation, since, unlike sweet whey, acid-salty whey have an adverse physiological effect, as demonstrated by Dantas28 in alfalfa seeds exposed to salt stress. Laynez-Garsaball29 observed a decrease in germination due to the effect of the increase in the osmotic potential exerted by NaCl, while Porta30 showed that the total inhibition of germination in high salt concentrations is due to the accumulation of chloride that decreases water absorption, affecting the germination rate. For their part, Mahdavi and Sanavy31 report that the ions present in whey can also cause hardening of cell walls, affecting seedling growth.
Navarro32 report the inhibition in the growth of hypocotyls of chicory, lettuce and endive seeds exposed to neutralized effluents from citrus and wine industries diluted at 103. If we compare this value with that obtained in the present work, in which inhibition was observed hypocotyl development at a concentration of 12% (101), then we can say that sweet whey has low toxicity.
Various authors such as Wang and Navarro32,33 report differences in tolerance to the toxicity of different substances in different species, with lettuce being one of the most used species in this type of study due to its high sensitivity. The tolerance of alfalfa can be explained by being a more tolerant seed at low pH, as has been reported by Marini34 who found that alfalfa can grow optimally between pH 5 and 6, but that it can tolerate even lower pH values, while the optimal pH for maize growth is between 6 and 7.2 and it presents growth inhibition in pH values less than 5.35
The differences observed between the experiments with seeds and seedlings indicate that the phytotoxicity is lower for alfalfa than for maize, and that in both species whey is more toxic in the early stages of development. On the other hand, it is necessary to carry out more research to evaluate what is the optimal concentration in other stages of the development of the plants studied, as well as the use of the cheese whey in field conditions.36-41
Laboratory tests show that sweet cheese whey has a low toxicity, with a high probability of being used safely in agricultural irrigation of species such as alfalfa and corn. Its use is recommended at concentrations less than 12% in order to maximize its use and at concentrations less than 4% as a plant growth promoter.
To the National Council of Science and Technology (CONACYT) for the financing of the doctoral scholarship in the Strategies for Regional Agricultural Development program at the Colegio de Postgraduados, Puebla.
The authors declare that there is no conflict of interest.

©2022 de, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.