Journal of
eISSN: 2373-437X


Research Article Volume 5 Issue 2
1Department of Microbiology and Parasitology, University of Buenos Aires, Argentina
2Department of Atomic Energy, National Commission of Atomic Energy (CNEA-Constituyentes), Argentina
Correspondence: Liliana Turcot, Department of Microbiology and Parasitology, School of Dentistry, University of Buenos Aires, Marcelo T de Alvear 2142, 2° Floor B, CP1122, Argentina , Tel 54 011 4964-1272
Received: June 14, 2017 | Published: June 21, 2017
Citation: Vilotta SM, Somaglia L, Bernat MI, Palacios N, Rosmino MF, et al. (2017) Effect of Human Serum on Biofilm Formation by Oral Mycoplasma on Nitinol Surfaces. J Microbiol Exp 5(2): 00142. DOI: 10.15406/jmen.2017.05.00142
Intoduction: Dental plaque harbors microorganisms that can enter the blood stream due to bacteremia, and reach heart prosthetic devices and become secondary septic foci. The presence of Mycoplasma in gingival-periodontal plaque and the ability of Mycoplasma to adhere to nitinol surfaces in cultures with horse serum have been documented. Human serum, a source of cholesterol, may promote Mycoplasma colonization of prosthetic devices.
Objective: To study the effect of human serum on biofilm formation by Mycoplasma on Nitinol® surfaces, using scanning electron microscopy (SEM).
Materials and methods: Sixty samples of nitinol were suspended in PPLO broth supplemented with 20% human serum, inoculated with Mycoplasma spp., and incubated under anaerobiosis for 5 days. The samples were then washed with sterile distilled water and processed for observation by SEM. Nitinol samples suspended in PPLO broth supplemented with 20% horse serum and inoculated with Mycoplasma spp. served as control.
Results: Mycoplasma spp. formed biofilms on all nitinol surfaces; the biofilms mostly consisted of cells undergoing cell division. Given a 95% confidence interval and a sample size of n=60, the lower confidence bound on the percentage of positive cases was 94%. The results showed that human serum enhanced biofilm formation by Mycoplasma on nitinol surfaces.
Keywords:mycoplasma, nitinol, human serum, biofilm
The formation of a biofilm is considered a strategy of bacteria to ensure their survival, for once they are embedded in this structure they are difficult to eradicate and are protected from antimicrobials and immune responses from the host. More than 80% of microbial infections in humans, including dental caries and gingival-periodontal diseases, are caused by bacteria organized in biofilms.1,2 Dental plaque harbors a wide variety of microorganisms. There are studies reported in the literature demonstrating the presence of Mycoplasma in subgingival plaque associated with periodontal disease.3˗5
Crespo et al.,6 determined the prevalence of Mycoplasma salivarium in biofilms of dental plaque associated with periodontal disease,6 as well as Mycoplasma adhesion to dental surfaces,7 Moreover, Ramella et al.,8 demonstrated the ability of Mycoplasma salivarium to form a biofilm on gingival explants.8 Vilotta et al.,9 demonstrated oral Mycoplasma spp. adhesion to biocompatible Nitinol® surfaces; the latter material is widely used in the manufacture of cardiovascular and orthodontic devices.9
Mycoplasmas genetically lack a cell wall and they are the smallest self-replicating organisms. The requirement of most mycoplasmas for cholesterol is unique among prokaryotes. Mycoplasmas require complex culture medium containing serum, which provides fatty acids and cholesterol for mycoplasma membrane synthesis.10
Dental procedures can cause bacteremias, resulting in the dissemination of microorganisms from the dental plaque and their potential adhesion to and colonization of cardio-vascular prosthetic devices, causing infection and resulting in need for device replacement.11 In a recent study, Henrich et al.,12 reported the finding of two inhabitants of the oral cavity in a biliary stent of a cholestatic patient: Mycoplasma salivarium, and Candida glabrata.
Taking into account the nutritional requirement of mycoplasmas for cholesterol, which is supplied by supplementing culture medium with horse serum, the spread of these microorganisms to prosthetic devices, and their ability to adhere to biocompatible surfaces, the aim of the present work was to study the effect of human serum on biofilm formation by Mycoplasma species on Nitinol® surfaces, using scanning electron microscopy (SEM).
The strain of Mycoplasma spp. used in the study was isolated from subgingival plaque collected from a site with chronic periodontitis. Sixty samples of nitinol were suspended individually in sterile PPLO broth supplemented with 20% heat inactivated human serum, and were inoculated with a suspension of Mycoplasma spp. The samples were incubated in an atmosphere consisting of 90% H2 and 10% CO2 at 37oC, for 5 days.
The samples were then washed with distilled water three times, fixed in 10% formol for 24 hours, dehydrated in increasing concentrations of alcohol, and metal-coated for observation by SEM (FEI Quanta 200 Microscope).
Nitinol samples suspended in sterile PPLO broth supplemented with 20% heat inactivated horse serum and inoculated with a suspension of Mycoplasma spp., and nitinol samples in sterile PPLO broth supplemented with 20% heat inactivated human serum not inoculated with Mycoplasma spp. served as controls.
SEM analysis showed that Mycoplasma spp. adhered to the nitinol surfaces forming a biofilm in all the studied samples (Figure 1 & 2). No microbial growth was observed on uninoculated control surfaces (Figure 3). Given a 95% confidence interval for a binomial distribution, and a sample size of n=60, the lower confidence bound on the percentage of positive cases was 94.00%.

Figure 1 Microphotograph showing nitinol surface inoculated with Mycoplasma spp and incubated in a culture medium containing horse serum for 5 days. Note the coccoid forms (c) and coccoid forms initiating reproduction (fr) (Orig. Mag: 20000X).
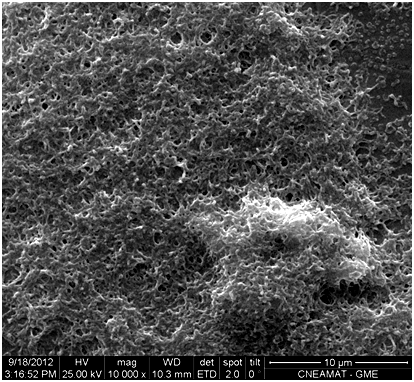
Figure 2 Microphotograph showing nitinol surface inoculated with Mycoplasma spp and incubated in human serum-containing medium during 5 days. Note growth in multilayers (Orig. Mag. 10000X).
Mycoplasmas found in control cultures supplemented with horse serum were predominantly cocci, 250 to 300 nm in diameter, and few cells were undergoing cell division (Figure 2). In contrast, most mycoplasmas cultured in human serum-supplemented medium were undergoing cell division, were predominantly filamentous and were approximately 1 µm long and 150-200 nm wide; they developed as a single layer or superimposed layers, and exhibited fewer cocci, which were approximately 200nm in diameter (Figure 2, 4-6).
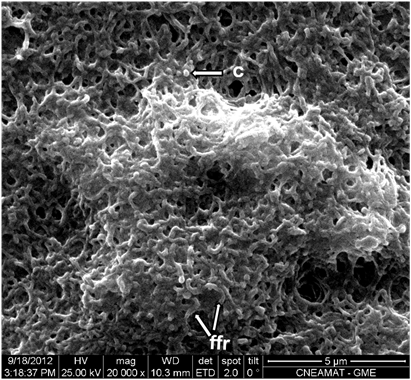
Figure 4 At higher magnification. A predominance of filamentous forms undergoing reproduction (ffr) and of coccoid forms (c) of Mycoplasma spp. can be observed (Orig. Mag: 20000X).
Bacteria in the form of biofilms have been recognized as important causes of a number of human infectious diseases, including dental caries and gingival-periodontal diseases. Given the adhesion and biofilm formation ability of oral bacteria, these microorganisms may be associated with the etiology and pathogenesis of not only oral infectious diseases but also diseases at distant sites caused by their systemic dissemination.13,14 The dental plaque is a reservoir of microorganisms that can enter the blood stream due to bacteremia and reach heart prosthetic devices, where they can become secondary septic foci and pose a risk to the patient.11,15
A number of research works have demonstrated that a variety of medical devices (including intravenous and arterial lines, urinary catheters, cardiac and biliary stents), can harbor biofilms that infect them, resulting in the need for device replacement.12,16 The present results show the ability of Mycoplasma to adhere to nitinol surfaces. In addition, they demonstrate that human serum increases growth and reproduction of this microorganism, enhancing biofilm formation on nitinol surfaces. Human serum would seem to act as an epigenetic factor that could regulate metabolic activity and consequently, the morphology, structure, and pathogenicity of Mycoplasma strains.
Human serum was used at a concentration of 20% because this is the concentration of horse serum used to supplement conventional Mycoplasma culture media.6
Average total serum cholesterol levels in the horse range from 80 to 180 mg/dl.17,18 In the present study, horse serum levels of cholesterol were 123 mg/dl, and those of human serum were 210 mg/dl. The difference in cholesterolemia between both sera may account for the increased growth of Mycoplasma in culture medium supplemented with human serum.
The choice of Nitinol® as an adhesion surface was based on the fact that microorganisms in the oral cavity can disseminate systemically and cause disease at distant sites and infection of cardio-vascular prosthetic devices, many of which are made of this biocompatible material.
Because ammonia is a pathogenic factor associated with the metabolic activity of mycoplasmas, it could be thought that the increased growth promoted by human serum may induce a greater pathogenic capacity in hypercholesterolemic patients. Thus, hypercholesterolemia would be a risk factor for complications associated with mycoplasma coinfections.
Further studies must be conducted in order to establish whether the percentage of cholesterol in human serum, over or below 200 mg/dl, affects the quantity of cells undergoing cell division. The results of the present study allow concluding that human serum promotes biofilm formation by Mycoplasma species on nitinol surfaces.
The authors wish to thank Dr. Macchi for his assistance in the statistical analyses and interpretation of the data. Grant: UBACYT 20020110200259
None.
None.

©2017 Vilotta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.