Journal of
eISSN: 2373-437X


Research Article Volume 10 Issue 3
1Conservation Preventive laboratory, National Archive of the Republic of Cuba, Havana city, Cuba
2Conservation Department, Provincial Historic Archive of Villa Clara, Santa Clara city, Cuba
Correspondence: Sofía Borrego, Conservation Preventive laboratory, National Archive of the Republic of Cuba, Compostela No. 906 esq. San Isidro, Habana Vieja, PO Box: 10100, Havana city, Cuba 8080, Tel +53 7862 9436, Fax +53 7866
Received: May 15, 2023 | Published: June 10, 2022
Citation: Borrego S, Molina A, Manso Y, et al. Distribution and diversity of the fungal pollution in repositories of the provincial historical archive of Villa Clara, Cuba. J Microbiol Exp. 2022;10(3):109-120. DOI: 10.15406/jmen.2022.10.00360
The aims of this study were 1) To characterize the mycobiota in four niches [indoor air from repositories (IA), outdoor air (OA), collected dust of indoor environments (CD) and documents surface (DS)] of the Provincial Historical Archive of Villa Clara, 2) To evaluate the settleable dust loadings, 3) To determine the similarity of the isolated taxa in the analyzed niches and 4) To predict the potential risk the isolated fungal species. Form indoor air of three repositories and the outdoor air were sampled in June, 2017 and March, 2018 using a SAS sampler and the indoor/outdoor (I/O) ratio was determined. The settled dust was collected passively during two years and the surface documents were sampled too. Sørensen's coefficient of similarity (QS) was calculated to compare the isolated taxa among the four studied niches. The extreme airborne fungal concentration in indoor environments was 515.5 CFU/m3 and the I/O ratios fluctuated from 1.0 to 1.5, evidencing a good environmental quality. The maximum settled dust load was 130.8 mg/m2/day with a top fungal concentration of 3x105 CFU/gdust, while the documents surface the highest concentration was 20 CFU/cm2. The obtained QSs were diverse: QS(IA-OA)=0.8-0.9, indicated a high species similarity; QS(IA-CD)=0.5-0.6, typical of moderate similarity; QS(IA-DS)=0.4, own of low similarity. This QS behavior showed that the repositories environment was strongly influenced by the outdoor environment. Aspergillus and Cladosporium were the predominant genera in indoor air, collected dust and documents surface. In these niches species predominance corresponding to the Aspergillus genus, in particular of the Flavi section was obtained. Some of the isolated species are opportunistic pathogens and toxigenic, and their concentrations were higher than the recommended, demonstrating the potential risk to which the archive personnel is exposed in a circumstantial way.
Keywords: airborne fungi, dustborne fungi, document surface fungi, archive, fungal pollution, indoor environments, environmental quality, opportunistic fungal pathogens
PHA VC, Provincial Historical Archive of Villa Clara; IA, indoor air from repositories; OA, outdoor air; CD, collected dust of indoor environments; DS, documents surface; QS, Sørensen's coefficient of similarity; I/O, indoor/outdoor; NARC, National Archive of the Republic of Cuba; RD, relative density; RF, relative frequency; PNSM, pigmented non-sporulating mycelia; WNSM, white non-sporulating mycelia
The continuous monitoring and control of environmental conditions in archives and libraries is one of the most important elements to preserve documents of heritage value. The prevalence of higher temperature (T), relative humidity (RH), inadequate lighting and ventilation together with high environmental dust concentrations or dust deposited on the documents and of microorganisms in the repositories air where the collections are kept, awakens every the attention of researchers and specialists in the documentary heritage conservation. These factors have been related to the integrity of the documents and the quality of life of the personnel who work in these institutions or come to them as users.1-4
Microclimatic and microbiological characteristics of the repositories are directly related to the area climatology, the year time, the architecture and building type, the premises size, the of ventilation or air conditioning type, the accumulated dusting level, the presence and staff number and even with the furniture location in the repositories.5,6 In Cuba there are regulations that establish the levels of T, RH, ventilation and lighting for the adequate documents conservation in different supports, as well as rules for the choice and location of the shelving, among others.7 However, factors such as the permissible sedimented dust load in indoor environments and the environmental microbiological quality in document repositories have not been regulated, mainly due to the lack of studies and the obtaining of data in a standardized way.
Among the biological agents that can cause documentation biodeterioration are microscopic fungi, which is why environmental fungal contamination of repositories where documents are kept is one of the main study objects for conservation scientists.8,9 However, other “niches”10 such as the collected dust5,11-13 and the documents surface14-18 have also been investigated. Some authors have even reported the importance of analyzing two or three niches (indoor air from repositories, collected dust in indoor environments, documents surface) for a correct characterization of the environment of a documents repository,6,12-19 regardless of whether it represents the basis for an adequate conservation strategy, in order to gather information to identify the specific danger that should lead to a prompt intervention providing correct conservation action.
Spores and other fungal propagules are among the largest groups of total biological material that is airborne and can have high biodeteriogenic and pathogenic potential.20-23 Fungi are capable of adapting to dissimilar environmental conditions and have powerful and versatile metabolic machinery supported by enzymes and other metabolites that allow them to use a large number of organic and inorganic substrates as nutrients. These characteristics make them potent biodeteriorating agents of cultural assets and in particular documentary heritage.14,24 They are, in fact, more influential in the paper documents biodeterioration than bacteria. Another important characteristic of fungi is that they have different structures and mechanisms of pathogenicity that cause specific diseases in humans. Its presence in indoor environments is associated with the triggering of respiratory and allergic conditions as well as infectious pathologies (mycoses) mediated by various virulence factors that vary depending on groups or taxa.3,25 Hence, many of these affections are among the so-called "Occupational Diseases".26 For this reason, it is necessary to perform systematic studies to characterize the environmental and microbiological conditions in the repositories and other archives premise, in order to diagnose and solve early problems associated with the triggering of biodeteriogenic pests in the collections and possible effects on the staff health. Therefore, the aims of this study were 1) to characterize the mycobiota in four niches [indoor air from repositories (IA), outdoor air (OA), collected dust of indoor environments (CD) and documents surface (DS)] of the Provincial Historical Archive of Villa Clara, 2) to evaluate the settleable dust loadings, 3) determine the similarity of the isolated taxa in the analyzed niches and 4) predict the potential risk that the isolated fungal species have for the personnel health.
Characterization of the analyzed repositories
The Provincial Historical Archive of Villa Clara (PHA VC) is located in one of the central provinces of the country (22º4'05.190'' N and 79º96'77.810'' W), 281.3 km (by road) from the city of Havana, Cuban capital (Figure 1a). The archive is situated in the historic center of the city, surrounded by avenues with high vehicular and pedestrian traffic and a few streets from the Bélico River, which provides a lot of humidity to the land and affects the building by capillarity, causing humidity to all the walls (Figures 1b and 1c).

Figure 1 Location of the province of Villa Clara on the map of Cuba. (A) The red balloon indicates the approximate position of the PHA VC. (B, C) They show the walls moisture in the PHA VC repositories. (D) Property sketch showing the repositories location and the sampling points. Area marked in light gray represents the archive ceiling, while the cement patio is not roofing. Points 1 and 2 with a black background indicate the points sampled outdoor of archive. Point 2 is located above the roof.
The property is made up of a large single-story house that has 4 repositories and other areas that are used as offices, a library and a reading room where services are provided. All repositories are located on the East side of the house. The right side (West) has a covered corridor, the cement patio with the cistern and there is a staircase that facilitates access to the house roof (Figure 1d). Of the repositories, only 3 were sampled, which were classified as repository 1 (R-1) belonging to the first repository, repository 2 (R-2) corresponding to repository 3 of the archive, and repository 3 (R-3) coinciding with the repository 4. R-1 has two windows, a very large one facing the house front and which is kept closed, and another small one located high up on the opposite wall, as well as the access door, which are kept open at all times to guarantee ventilation inside. R-2 only have a medium window and the entrance door to the premises that remain open during the working day, while R-3 has 3 windows and the door that also remain open to guarantee natural ventilation and air circulation in its inside. The repositories dimensions (length x width x height) are: R-1=6 m x 3 m x 4 m, R-2=5 m x 3 m x 4 m, and R-3=6 m x 3.20 m x 4 m.
This archive preserves 34 documentary funds that cover the period between the 17th century and the present day, being the Capitulary Acts and the notes on the council operation the oldest documents. All these documents are on paper and some Notarial Protocol bindings are made of parchment.
Airborne fungal sampling
Two samples were made to determine airborne fungi. The first sampling performed on June 27, 2017, a day corresponding to the rainy season in Cuba or summer, while the second sampling was made on March 28, 2018, a day that belongs to the season of little rains or winter.
Three points in each repository were sampled in triplicate according to Sanchis27 and in the outdoor two points were sampled (Figure 1d); one of them in the roof and the other in the archive courtyard. In the air sampling a SAS biocollector (Super 100TM, Italy) was used with an air flow rate of 100 L/min for 2 min at a height of 1.5 m. The culture medium used were Malt Extract Agar (MEA) (BIOCEN, Cuba) supplemented with 7.5% sodium chloride (MEA + NaCl)9,28 and MEA at pH = 5.9,29 After sampling, the Petri dishes were incubated inverted for 5 to 7 days at 30°C. Later, the colonies were counted and the colony forming units per m3 of air (CFU/m3) were calculated according to the instructions described in the equipment manual.30
The indoor/outdoor ratio (I/O) was calculated according to Aquino and Góes Siqueira31 who indicated that if this ratio is equal to or less than 1.5, then the environments are not polluted because they have good ventilation and air circulation in the indoor the places. Together with the microbiological samples, temperature (T) and relative humidity (RH) were measured at the same analyzed points using a digital thermo hygrometer Pen TH 8709 (China).
Determinations of the settled dust and its fungal load
A passive method was used to sample for the settling of the dry dust. For this purpose, previously weighed sterile 110 mm plastic Petri dishes were placed open during the 2 years of the study. They were placed on the shelves (approximately 2.5 m high) coinciding with the same points where the air samples were taken. Thus, the dust was deposited on the surface of the open plastic dishes in the same way that it does on the documents.13,32 During the capture of the dust collectors, they were closed with their corresponding covers and were transported to the laboratory. Subsequently, they were placed in desiccators with silica gel and weighed every 24 h until the collectors reached constant weight.
The determination of the total load of settled dust was carried out according to the formula proposed by Oliva et al.,33 and the deposition rate was calculated as mg/m2/day.
Total dust load = (Pf - Pi)/A x t
Where: Pf- final dry weight of the Petri dish with powder, Pi- initial dry weight of the sterile Petri dish, A- area of the dish (m2), t- time (days)
For microbiological sampling of the dust, 0.01 g of the settled dust was taken from each collector and 0.5 mL of sterile distilled water was added. Samples were allowed to stand for 1 h and each sample was randomly shaken well at intervals for 45min. Serial dilutions were then made and the selected ones were seeded in 110 mm Petri dishes containing the culture media MEA + NaCl and MEA at pH 5. Dishes were invert incubated for 5 to 7 days at 30°C. After the incubation, the colony forming units were counted in order to determine the fungal concentration expressed in colony forming units per g of collected dust (CFU/g).13
CFU/g = (Number of total CFUs obtained x dilution)/0.01 g of collected dust
Sampling of the documents surface
Twelve historical paper documents were analyzed. They are notarial protocols dating from the first half of the 20th century (1905-1957). The documents were apparently clean; however, they were carefully examined with the help of a 30X magnifying glass and no fungal growth was observed on them. The samples were collected in a 4cm2 area using sterile cotton swabs moistened with sterile distilled water, which were placed in by separate in a sterile tube and they were transported to the laboratory. To each tube, 1 ml of sterile physiological solution was added and they were thoroughly shaken at random intervals for 45 minutes;18,34 then serial dilutions were made. Of the selected dilutions for each sample, 0.1ml was inoculated in Petri dishes with the media MEA + NaCl and MEA at pH=5. Subsequently, the dishes were incubated inverted at 30°C for 5 to 7 days. Fungal concentration was reported in CFU/cm2.
Fungal identification
For the identification of fungal isolates, the cultural and morphological characteristics of the colonies, as well as conidiophores, conidia and other structures of interest, were observed under a stereomicroscope (X14) and a trinocular clear field microscope (Olympus, Japan) at X40 and X100 coupled to a digital camera (Samsung, Korea). Different mycological key manuals were used35-42 and MycoBank website was also consulted.
Ecological approaches
The relative density (RD) of fungal genera isolated from the indoor air of each repository was calculated according to Smith,43 where:
RD = (number of colonies of one taxon / total number of colonies) x 100
The relative frequency (RF) of fungal species detected on documents surface and those detected in the three studied niches (indoor air, collected dust and documents surface) was determined according to Esquivel et al., 44 where:
RF = (times a specie is detected / total number of sampling realized) x 100
The ecological categories are classified as: Abundant (A) with RF = 100-81%, Common (C) with RF = 80-61%, Frequent (F) with RF = 60-41%, Occasional (O) with RF = 40-21%, Rare (R) with RF = 20-0%.28
Sørensen's coefficient of similarity (QS) was used to compare the similarities of the taxa obtained among the four ecological niches analyzed (indoor air, outdoor air, documents surface and collected dust). The comparisons made were between indoor and outdoor air, indoor air and documents surface, indoor air and dust as well as documents and dust collected.13,29
QS = 2a/b + c
Where: a- is the number of common genera detected in the two niches being compared, b- the number of taxa detected only in one niche and c- the number of taxa detected in the other niche.
The QS values are given in the range from 0-1. A value equal to 0 indicates that the taxa obtained in both compared niches are completely different and a value equal to 1 indicates that the taxa are identical.45
Statistical analysis
The data obtained were analyzed using the statistical program the Statgraphics Centurion XV. Student test was used to compare the T and RH averages obtained in 2017 and 2018, while to compare the dust total load collected on the indoor environments and their fungal concentrations an ANOVA was used followed by the Fisher LSD test (Least Significant Difference).
Concentration of airborne fungi in the repositories environments
Most of the research on airborne fungi in Cuban archives, libraries and museums has been performed in institutions located in the Havana city (the country's capital) since most of these institutions are located in this city, hence this is the first investigation performed in an archive of the central region of the country.
In order to have a greater representativeness of the concentration and diversity of airborne fungi in a given environment, it is important to carry out samplings at different times of the year for as long a period as possible. Cuba is characterized by having two seasons in the year, one is the rainy season (summer) that runs from June 1 to November 30, the other season is winter and is characterized by little rains and duration from 1 December and until May 31. Hurricanes usually occur during the summer months and have a greater impact on the western region of the country. The winter season is more intense in the west and center of the country. The values of T and RH are high throughout the year, the average annual T is 28ºC and the RH is 80% approximately, but there are differences between the west and center of the country with respect to the east, where the T is always higher, while the RH is usually higher in the western and central regions.46 For these reasons, natural cross ventilation plays an important role in the freshing of spaces in an ecological way, it contributes to lowering the environmental RH and thereby reducing the airborne bioparticles deposition, specially the fungal propagules, an aspect that helps to slow down the growth microbial, particularly the fungal growth, atop the different materials preserved in archive repositories (documents, shelves, etc.).
In the first sampling made in June 27, 2017, fungal concentrations ranged between 341.9 and 363.6 CFU/m3, while in the second sampling (March 28, 2018) the values fluctuated between 350.6 and 515.5 CFU/m3. It should be noted that although there were no statistically significant differences, R-3 showed the highest value in both isolates (Table 1). On the other hand, fungal concentrations in outdoor air were very similar in both samplings (357 and 350 CFU/m3, respectively). As can be seen, despite having carried out the samplings in two different seasons, the fungal concentrations detected in the indoor environments were very similar, and these in turn were very like to the concentrations found outdoors, which shows that in the repositories the characteristics of environmental mycobiota are quite stable despite being a building with high humidity in the walls; but apparently its stability is given by the natural ventilation that the repositories have, which allows a correct exchange of air with the outside and an adequate circulation of the air inside them.
|
Repository |
1st Sampling |
2nd Sampling |
||||||||
|
Indoor concentration (CFU/m3) |
Outdoor concentration (CFU/m3) |
I/O |
T (°C) |
RH (%) |
Indoor concentration (CFU/m3) |
Outdoor concentration (CFU/m3) |
I/O |
T (°C) |
RH (%) |
|
|
R-1 |
352 |
- |
1 |
29.1 |
67.5 |
357.4 |
- |
1 |
27.3 |
53 |
|
R-2 |
341.9 |
- |
1 |
29.2 |
67.7 |
350.6 |
- |
1 |
26.6 |
51.2 |
|
R-3 |
363.6 |
- |
1 |
29.4 |
71.2 |
515.5 |
- |
1.5 |
26.5 |
56.2 |
|
Average |
352.5 |
357 |
- |
29.2 |
68.8 |
407.8 |
350 |
- |
26.8 |
53.5 * |
Table 1 Airborne fungi concentrations obtained in indoor and outdoor air as well as indoor/outdoor ratios in each studied repository in the two performed samplings
*: Indicates significant difference according to Student's t test (P ≤ 0.05) when the RH were compared.
Though there is no international consensus to indicate when an environment is contaminated or not, some authors have pointed out that in order to know if an environment has good quality, it is important to compare the obtained indoor concentrations with respect to the outdoor ones to analyze the I/O ratio and depending on from the value obtained, the environmental microbiological quality can be estimated.12,16,31,47,48 Consequently, simultaneous determinations of the outdoor air were made in each sampling. The I/O ratio in the first sampling was 1 for all repositories, while in the second only R-3 showed a value of 1.5, indicating that all of them have a low level of contamination and a good environmental quality.31 The I/O ratios obtained turned out to be quite stable regardless of the time of sampling, which shows that the natural ventilation in the repositories is good and that it guarantees good air circulation inside these spaces. This advantageous aspect had a different behavior in the PHA of Pinar del Río, an archive located in the western region of the country9 and in the PHA of Santiago de Cuba, situated in the eastern region.49
Regarding T and RH, very similar values were obtained among the repositories. In the first sampling (rainy season of 2017) the values of T ranged between 29.1 and 29.4°C averaging 29.2°C, while in the second sampling (little rains season of 2018) the values fluctuated between 26.5 and 27.3°C with an average of 26.8°C. The RH obtained in 2017 showed values between 67.5 and 71.2% with an average of 68.8%, while in the second sampling (2018) the percentages varied from 51.2 to 56.2% and averaged 53.5%, a value that is significantly lower than that obtained in the first sampling.
Diversity of the fungal species isolated from the repositories air
About taxa detected on indoor air of the repositories, a predominance of anamorphs of the phylum Ascomycota was evidenced (Figure 2), which was in accordance with the previous report.9,16,49-51 This prevalence may be due to the fact that representatives of this phylum have been detected at high concentrations in the outdoor air of Cuba.49 A total of 10 genera and two non-sporulating mycelia were identified, one white (WNSM) and other pigmented (PNSM). Of them, 8 genera were detected in the first sampling and 5 genera were isolated in the second isolation, and non-sporulating mycelia were found in both samplings. In the two isolations a marked predominance of the Aspergillus and Cladosporium genera was detected, a result that agrees with others previously obtained in Cuban archives.9,28,34,52-54 However, Penicillium, a genus that has been predominantly detected in other environments of Cuban archives49,52-54 and in libraries, archives and museums of other world regions,50,51,55-57 was only detected in the first sampling. Fusarium, Nigrospora, Rhizopus, Rhodotorula and Talaromyces genera were also detected in the first sampling, while Chrysosporium, Neurospora and Nigrospora genera were detected in the second isolation. Although most of these genera have been previously detected in the environment of other Cuban archives53,54 and other countries,17,56,58 it should be noted that Talaromyces is the second time it has been detected in the repositories' air of Cuban archives; the first time, it was isolated from the repositories environment of the Provincial Historical Archive of Santiago de Cuba.49
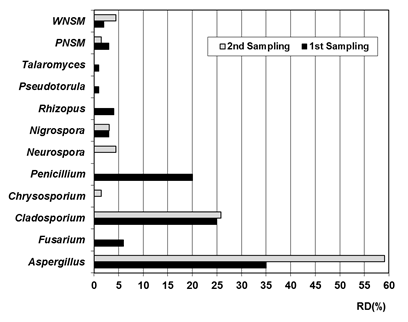
Figure 2 Relative density (RD) of the genera and non-sporulating mycelia detected in the indoor air of the PHA VC repositories. PNSM: pigmented non-sporulating mycelium. WNSM: white non-sporulating mycelium.
Figure 3 shows the concentrations of the different species isolated in each sampling. As can be seen, in the first sampling a total of 20 species and two non-sporulating mycelia were detected, but from the three repositories only 11 similar species (55% of the total) were isolated at different concentrations and were: A. flavus and A. oryzae (both species belonging to the Flavi section), Cl. cladosporioides, Cl. funiculosum, Cl. herbarum, Cl. sphaerospermum, Fusarium nivale, Nigrospora oryzae, P. chrysogenum, P. citrinum and Rhizopus stolonifer. In addition to them, in R-1 and R-2 the species Fusarium semitectum and Nigrospora sphaerica were isolated. In R-1 the species P. janczewskii, P. rugulosum and a pigmented mycelium (PNSM) were also detected, while in R-3 were isolated the species A. ornatus, A. parasiticus, P. brevicompactum and Pseudotorula sp. It can also be seen in this figure that 16 species and a non-sporulating mycelium were isolated from the outdoor air. In the second sampling, a total of 16 species and two non-sporulated mycelia were detected, but from the three repositories only 5 similar species (37.5% of the total) and one white mycelium were isolated; the species were A. flavus, A. niger, A. versicolor, Cl. cladosporioides, Cl. oxysporum. The species A. ornatus, A. penicillioides and Chrysosporium merdarium were also isolated from the environment of R-1. From the air of R-2, the species A. glaucus, A. oryzae, Cl. funiculosum and Cl. sphaerospermum were isolated. From R-1 and R-2, the species Cl. tenuissimum, Nigrospora oryzae and PNSM were also spotted. From R-1 and R-3 the species A. penicillioides was also isolated, while from R-2 and R-3 the species A. restrictus was isolated. In addition, Neurospora crassa species was isolated from R-3. A total of 11 species and one white mycelium (WNSM) were isolated from outdoor air.


Figure 3 RelativeConcentrations of species and the non-sporulating mycelia detected in the indoor and outdoor air of the PHA VC repositories. PNSM, pigmented non-sporulating mycelium; WNSM, white non-sporulating mycelium.
It is noteworthy that most of the species isolated in the repositories environments as well as the non-sporulating mycelia were detected in the environment outdoor the archive, which demonstrates the incidence of the outdoor environment in the interiors due to the natural ventilation existing in them. On the other hand, many of these species have been previously detected in environments of Cuban archives.9,13,28,34,49,53,54,59 Their detection may be due to the fact that many of them require relatively low water activity (aw=0.70-0.79),58 favoring their existence in environments with certain levels of dust, as is the case in this archive.
Although in Cuba there are no regulations that indicate the permissible levels of the species in indoor environments, in other countries there are regulations that establish quality thresholds. Such is the case of Portugal,58 which has established that toxigenic species such as A. flavus, A. fumigatus, A. versicolor and others should not have more than 12 CFU/m3 or that uncommon species should not exceed the 50 CFU/m3. Likewise, Canada has established that the concentration of each species should not exceed 50 CFU/m3 as long as these species do not correspond to the genera Cladosporium or Alternaria.60 Hence, in this study those species with concentrations equal to or greater than 50 CFU/m3 and their I/O ratios were taken into account.
Table 2 shows the species detected in indoor air at concentrations ³50 CFU/m3. As can be seen in the first sampling, only three species showed high concentrations and they were A. flavus with 59.5 CFU/m3 in R-1 and 50 CFU/m3 in R-2, Cl. cladosporioides with 77.5 CFU/m3 in R-1, 112.6 CFU/m3 in R-2 and 114.4 CFU/m3 in R-3 as well as Cl. herbarum with 50 CFU/m3 in R-2 and 68.5 CFU/m3 in R-3. When the I/O ratios of these species were compared, values ranging between 0.8 and 1.7 were obtained. In the second sampling, a greater number of species were detected with concentrations ≥50 CFU/m3. In this case, 8 species showed this behavior with values that ranged between 54.1 and 135.1 CFU/m3 and they were A. flavus, Cl. cladosporioides, Cl. oxysporum, Cl. tenuissimum, Cl. sphaerospermum, A. penicillioides, A. restrictus and N. crassa. When comparing the I/O ratios, values ranging between 0.9 and 9.0 were obtained; however, in the case of Cl. tenuissimum the ratio was 54.1, an extremely high value.
|
Repository |
1st Sampling |
2nd Sampling |
||||||
|
Specie |
Indoor concentration (CFU/m3) |
Outdoor concentration (CFU/m3) |
I = I/O |
Specie |
Indoor concentration (CFU/m3) |
Outdoor concentration (CFU/m3) |
I = I/O |
|
|
R-1 |
A. flavus |
59.5 |
35 |
1.7 |
A. flavus |
66.1 |
50 |
1.3 |
|
Cl. cladosporioides |
77.5 |
100 |
0.8 |
Cl. cladosporioides |
135.1 |
100 |
1.3 |
|
|
- |
- |
- |
Cl. oxysporum |
54.1 |
20 |
2.7 |
||
|
- |
- |
- |
Cl. tenuissimum |
54.1 |
0 |
54.1 |
||
|
R-2 |
A. flavus |
50 |
35 |
1.4 |
A. flavus |
68.9 |
50 |
1.4 |
|
Cl. cladosporioides |
112.6 |
100 |
1.1 |
Cl. cladosporioides |
103.6 |
100 |
1 |
|
|
Cl. herbarum |
50 |
40 |
1.3 |
Cl. sphaerospermum |
54.1 |
33 |
1.6 |
|
|
R-3 |
Cl. cladosporioides |
114.4 |
100 |
1.1 |
A. flavus |
135.1 |
50 |
2.7 |
|
Cl. herbarum |
68.5 |
40 |
1.7 |
A. penicillioides |
90.1 |
22 |
4.1 |
|
|
- |
- |
- |
A. restrictus |
90.1 |
10 |
9 |
||
|
- |
- |
- |
Cl. cladosporioides |
90.1 |
100 |
0.9 |
||
|
|
- |
- |
|
- |
N. crassa |
54.1 |
8 |
6.8 |
Table 2 Species of airborne fungi obtained in each repository by sampling at concentrations 50 CFU/m3 and their I/O ratios
The fact that the I/O ratio of many species was higher than 1.5 could be due to two reasons: 1) the presence of an internal source of contamination not identified by the archive curators and 2) the effect of settled dust. Such is the case of the species A. flavus, Cl. herbarum, Cl. oxysporum, A. restrictus, N. crassa, and the most significant Cl. tenuissimum that were detected in the collected dust (Figure 4).
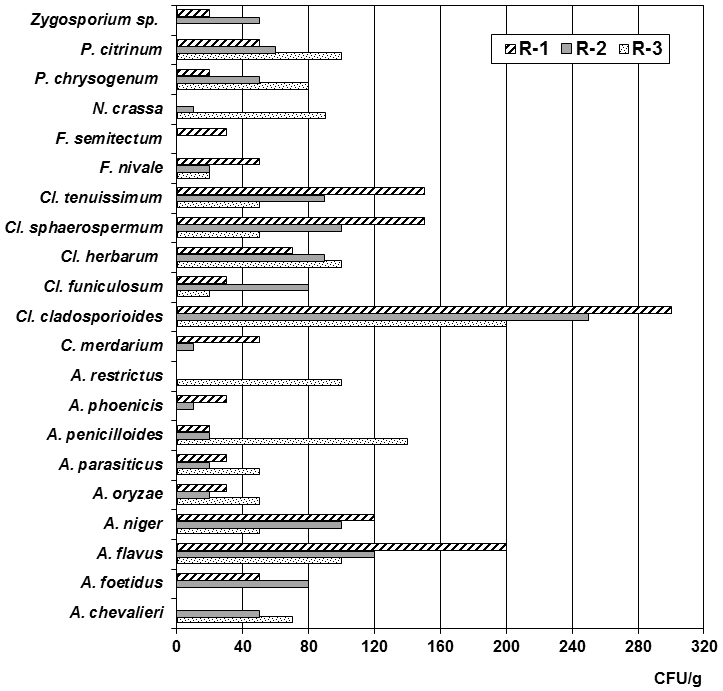
Figure 4 Concentrations of isolated species from the collected dust on indoor of each studied repository.
Settleable dust loadings and fungal concentrations in the studied repositories
Environmental dust has a complex and qualitatively and quantitatively variable composition32 that depends, among other factors, like the direction and speed of the winds, the location and size of the place studied, and the season of the year. Its components include microorganisms, soluble and insoluble salts in water, carbon particles and metals as traces, fatty acids and other solid or liquid organic compounds2,61 constituting a true chemical arsenal with a high capacity to cause damage to documents, among which can be mentioned dirt, abrasions, stains, yellowing, brittle appearance of the paper, etc.2,11,62
The dust load collected was different in each of the studied repositories. In R-1 the value was very low (4 mg/m2/day), in R-2 the load was slightly higher (9.3 mg/m2/day), but in R-3 the value was significantly high (130.8 mg/m2/day) (Table 3). In all cases, the dust load was higher than those reported by Awad et al.12 in a study performed in a museum in Cairo, Egypt, where they detected very low loads in the repositories (0.1-1.2 mg/m2/day).
|
Repository |
Total load of settled dust (mg/m2/days) |
Fungal concentrations (CFU/g) |
|
R-1 |
4.0 a |
5.5 x 102 a |
|
R-2 |
9.3 b |
4.1 x 102 a |
|
R-3 |
130.8 c |
3.0 x 105 b |
Table 3 Dust total load collected on indoor environments of the studied repositories and fungal concentration detected in them
a,b,c: Indicates significant differences according to the LSD test (P ≤ 0.05) when comparing each determination among the different repositories. Each determination was made at 3 points in each repository and the data were averaged (n=3).
It is noteworthy that the dust load obtained in R-3 was much higher than that reported by Rodríguez11 in a study made in the Documentary Center of the National Museum of Music in Cuba (64 mg/m2/day) or the reached in the repositories of NARC where a maximum load of 22.8 mg/m2/day was obtained.13 This behavior may be due to construction problems in the repository caused by the high humidity in its walls (the water rises by capillarity), which makes the ceiling and walls plaster come off easily and this has contributed to the high levels of settled dust.
The fungal concentrations obtained in R-1 and R-2 were similar to those previously reported by Borrego et al.13 who detected concentrations that varied between 102 and 103 CFU/g. However, the fungal concentration obtained in R-3 was higher (3.0 x 105 CFU/g) and was similar to the values reported by Molina63 and Rodríguez11 who revealed concentrations in the order of 105 CFU/g respectively (6 x 105 CFU/g at the NARC as well as 1.2 x 105 and 2.1 x 105 CFU/g at the Documentation Center of the National Music Museum). However, it is suggested that the fungal concentration in the dust can vary from 6 x 103 to 7 x 107 CFU/g of dust.64 The fungal concentrations detected in this study were markedly lower than those reported by OSHA,65 which established limit concentrations in the order of 106 CFU/g of dust. On the other hand, it has been shown that the dust contains bacteria and a large number of fungal propagules61,66 that can even reach significantly high concentrations (up to 107 CFU/g of dust),67 constituting a reservoir of fungal contamination; hence, dust sample analyzes have been suggested to provide a better indication of cumulative fungal exposures than short-term air samples,68 making them an important source of information when performing microbiological diagnosis in an indoor environment.69,70
Fungal taxa distribution in the settled dust
A total of 20 species were isolated from the collected dust, distributed as follows: 9 were from the Aspergillus genus, 5 were from Cladosporium, 2 belonged to Penicillium and 4 corresponded to other genera (Figure 4). Thirteen species (65%) were isolated from the dust collected in the three repositories and they were A. flavus, A. oryzae y A. parasiticus (section Flavi), A. niger (section Nigri), A. penicillioides (section Restricti), Cl. cladosporioides, Cl. funiculosum, Cl. herbarum, Cl. sphaerospermum, Cl. tenuissimum, F. nivale, P. chrysogenum y P. citrinum. From the dust collected in R-1 and R-2 were also isolated the species A. foetidus, A. phoenicis, Chrysosporium merdarium and Zygosporium sp. From the dust collected in R-1 F. semitectum species was detected, too. In the dust that was collected in R-2 and R-3 A. chevalieri and N. crassa species were detected, while A. restrictus was also isolated from the dust collected in R-3.
Species of the Aspergillus genus showed an important ecological impact on the collected dust. Species from the Flavi (A. flavus, A. oryzae, A. parasiticus) and Nigri (A. foetidus, A. niger, A. phoenicis) sections predominated, followed by species from sections Aspergillus (A. chevalieri, A. glaucus) and Restricti (A. penicillioides, A. restrictus). It is noteworthy that some of them, such as A. penicillioides and A. restrictus, are xerophilous,71 so finding them in dust would not be unusual due to the characteristics of this matrix.62
Many of the species of Aspergillus, Cladosporium and Penicillium obtained in this study have previously been detected in the dust collected in Cuban archives.11,13,63 However, Nevalainen & Morawska72 reported data on fungal genera and species detected in a large number of microbiological studies performed in this ecological niche in much of the world, and informed more than 20 genera and 200 species, among which are those detected in this study. In this way, the settled dust, on the one hand, constitutes a potential danger for collections of heritage value69 since it hydrates when the RH increases, and this favors the conditions for fungi to develop and establish themselves on the documentary supports where they have settled and, on the other hand, constitute a reservoir of allergens and other elements that can be harmful to health.61,73
Fungal concentration and diversity on analyzed documents
Microbial concentration values on surfaces may be different from those values obtained from the indoor air. Therefore, the results from surface sampling cannot be directly associated with microorganisms found in the air.74 However, the microbiological control of surfaces is a valid way to determine a possible source of biocontamination. Actually, surface sampling is recommended as a complementary test to air sampling, being a way to detect fungi in indoor environments.70 On the other hand, it is known that at an RH of 65% and a T higher than 20ºC, the moisture content of the paper can increase by 8 and 10% and these conditions can favor fungal development.75 For this reason, the sampling of the documents surface preserved in the same repositories where the air and the dust settled for months were analyzed was performed.
The fungal load detected in the documents was low in general, regardless of the time of sampling. The concentrations ranged from 4 to 20 CFU/cm2, indicating that the documents were in fairly good hygienic condition (Table 4), an issue that the conservators guarantee through a Preventive Conservation Plan that they have designed in the archive that includes quick surface cleaning of documents monthly with vacuum cleaners. These results are contrary to studies previously performed at the NARC where the concentrations detected on the documents have ranged between 0.2 x 10 and 2.5 x 103 CFU/cm2 18,34,63,76 as well as studies performed in Polish archives and libraries where fungal concentrations on surfaces documents fluctuated between 1 x 102 and 2 x 105 CFU/cm2.77
|
Repository |
1st sampling |
2nd sampling |
||
|
Document |
CFU/cm2 |
Document |
CFU/cm2 |
|
|
R-1 |
D-1 |
12 |
D-7 |
10 |
|
D-2 |
14 |
D-8 |
11 |
|
|
R-2 |
D-3 |
20 |
D-9 |
9 |
|
D-4 |
10 |
D-10 |
10 |
|
|
R-3 |
D-5 |
5 |
D-11 |
12 |
|
|
D-6 |
4 |
D-12 |
8 |
Table 4 Fungal concentrations detected from the analyzed documents surface
From documents, four genera were isolated, being Aspergillus (60%) and Penicillium (20%) the predominant ones, followed by Cladosporium (13.3%) and Talaromyces (6.7%), which were detected in a smaller proportion. Similar behavior was previously detected in studies made from documents of NARC18,34,63 and the sampled documents in other countries.14,15,17 Of these, a total of 15 species were detected, 9 Aspergillus species, 3 of Penicillium, 2 of Cladosporium and 1 of Talaromyces. But the species that showed a greater ecological impact were A. niger, Cl. sphaerospermum and P. chrysogenum, which were also detected in the air of the three studied repositories (Table 5). Although Aspergillus species belonging to the Nigri section (A. foetidus, A. niger, A. phoenicis) predominated, not all of them showed the same ecological impact. As shown in the table, A. niger turned out to be a frequent species, while A. foetidus and A. phoenicis were rare species. However, Cl. sphaerospermum and P. chrysogenum were the species detected in the largest number of documents (6 of them, equivalent to 50%). Many of these species were previously isolated from documents preserved in NARC34,76 and in other countries.78-80
|
No. |
Species |
Section * |
Sampled documents |
RF (%) |
EC |
|
1 |
A. candidus Link |
Candidi |
D-1 |
8.3 |
R |
|
2 |
A. chevalieri L. Mangin |
Aspergillus |
D-4, D-6 |
16.7 |
R |
|
3 |
A. foetidus Thom y Raper |
Nigri |
D-2, D-4 |
16.7 |
R |
|
4 |
A. flavus Link |
Flavi |
D-2, D-4 |
16.7 |
R |
|
5 |
A. niger Tiegh. |
Nigri |
D-1, D-7, D-9, D-11, D-12 |
41.7 |
F |
|
6 |
A. parasiticus Speare |
Flavi |
D-5, D-6 |
16.7 |
R |
|
7 |
A. penicillioides Spegazzini |
Restricti |
D-7, D-8, D-11, D-12 |
33.3 |
O |
|
8 |
A. phoenicis (Corda) Thom |
Nigri |
D-1 |
8.3 |
R |
|
9 |
A. restrictus G. Smith |
Restricti |
D-8, D-11, D-12 |
25 |
O |
|
10 |
Cl. cladosporioides (Fresen) G.A. de Vries |
D-5, D-7, D-10 |
25 |
O |
|
|
11 |
Cl. sphaerospermum Penz. |
D-1, D-2, D-4, D-5, D-6, D-10 |
50 |
F |
|
|
12 |
P. chrysogenum Thom |
D-2, D-3, D-6, D-9, D-10, D-12 |
50 |
F |
|
|
13 |
P. citrinum Thom |
D-1, D-2, D-3, D-4 |
33.3 |
O |
|
|
14 |
P. janczewskii K.W. Zaleski |
D-1, D-2 |
16.7 |
R |
|
|
15 |
Talaromyces sp. C.R. Benj. |
D-4, D-6, D-8 |
25 |
O |
|
Table 5 Fungal species detected on the documents surface (n = 12) and their ecological impacts
*: Indicates that it is only valid for Aspergillus species. Ecological categories (EC) according to Relative frequency (RF): Abundant taxa (A) were those that had a RF=100-81%, Common taxa (C) had a RF=80-61%, Frequent taxa (F) had a RF=60-41%, Occasional taxa (O) had a RF=40-21% and Rare taxa (R) had a RF=20-0.1%.
Species similarity in the four studied niches (IA: indoor air, OA: outdoor air, CD: collected dust, DS: documents surface)
Table 6 shows the species isolated in 1) the IA of the repositories by sampling, 2) from the CD and 3) those detected on the analyzed DS. As can be seen, there was a predominance of species corresponding to the Aspergillus genus (36.1%) followed by species of Cladosporium (16.7%). Of the Aspergillus genus, the species corresponding to the Flavi section showed the greatest ecological impact, since they turned out to be abundant or common (A. flavus, A. oryzae and A. parasiticus). The species of the Nigri section (A. foetidus, A. niger, A. phoenicis) although important, had a lower ecological impact, since they were species that turned out to be common to frequent. The other two abundant species were Cl. cladosporioides and Cl. sphaerospermum followed by the common species Cl. funiculosum, P. chrysogenum and P. citrinum.
|
Specie/mycelium |
Section * |
Indoor Air (IA) |
Collected Dust (CD) |
Documents Surface (DS) |
RF (%) |
EC |
|
|
1st sampling |
2nd sampling |
||||||
|
Aspergillus candidus Link |
Candidi |
- |
- |
- |
+ |
25 |
O |
|
A. chevalieri L. Mangin |
Aspergillus |
- |
- |
+ |
+ |
50 |
F |
|
A. foetidus Thom y Raper |
Nigri |
- |
- |
+ |
+ |
50 |
F |
|
A. flavus Link |
Flavi |
+ |
+ |
+ |
+ |
100 |
A |
|
A. glaucus Link |
Aspergillus |
- |
+ |
- |
- |
25 |
O |
|
A. niger Tiegh. |
Nigri |
- |
+ |
+ |
+ |
75 |
C |
|
A. ornatus Raper, Fennell & Tresner |
Ornati |
+ |
+ |
- |
- |
25 |
O |
|
A. oryzae (Ahlb.) Cahn |
Flavi |
+ |
+ |
+ |
- |
75 |
C |
|
A. parasiticus Speare |
Flavi |
+ |
- |
+ |
+ |
75 |
C |
|
A. penicillioides Spegazzini |
Restricti |
- |
+ |
+ |
+ |
75 |
C |
|
A. phoenicis (Corda) Thom |
Nigri |
- |
- |
+ |
+ |
50 |
F |
|
A. restrictus G. Smith |
Restricti |
- |
+ |
+ |
+ |
75 |
C |
|
A. versicolor (Vuill.) Tiraboschi |
Versicolor |
- |
+ |
- |
- |
25 |
O |
|
Chrysosporium merdarium (Link) J.W. Carmich. |
- |
+ |
+ |
- |
50 |
F |
|
|
Cladosporium cladosporioides (Fresen) G.A. de Vries |
+ |
+ |
+ |
+ |
100 |
A |
|
|
Cl. funiculosum W. Yamam. |
+ |
+ |
+ |
- |
75 |
C |
|
|
Cl. herbarum (Pers.: Fr.) Link |
+ |
- |
+ |
- |
50 |
F |
|
|
Cl. oxysporum Berk. & M.A. Curtis |
- |
+ |
- |
- |
25 |
O |
|
|
Cl. sphaerospermum Penz. |
+ |
+ |
+ |
+ |
100 |
A |
|
|
Cl. tenuissimum Cooke, Grevillea |
- |
+ |
+ |
- |
50 |
F |
|
|
Fusarium nivale (Fr.) Sorauer |
+ |
- |
+ |
- |
50 |
F |
|
|
F. semitectum Berk. & Ravenel |
+ |
- |
+ |
- |
50 |
F |
|
|
Neurospora crassa Shear & B.O. Dodge |
- |
+ |
+ |
- |
50 |
F |
|
|
Nigrospora oryzae Hudson |
+ |
+ |
- |
- |
50 |
F |
|
|
N. sphaerica (Sacc.) E. W Mason |
+ |
- |
- |
- |
25 |
O |
|
|
Penicillium brevicompactum Dierckx |
+ |
- |
- |
- |
25 |
O |
|
|
P. chrysogenum Thom |
+ |
- |
+ |
+ |
75 |
C |
|
|
P. citrinum Thom |
+ |
- |
+ |
+ |
75 |
C |
|
|
P. janczewskii K.W. Zaleski |
+ |
- |
- |
+ |
50 |
F |
|
|
P. rugulosum Thom |
+ |
- |
- |
- |
25 |
O |
|
|
Rhizopus stolonifer (Ehrenb.) Vuill. |
+ |
- |
- |
- |
25 |
O |
|
|
Talaromyces sp. C.R. Benj. |
+ |
- |
- |
+ |
50 |
F |
|
|
Pseudotorula sp. Subram. |
+ |
- |
- |
- |
25 |
O |
|
|
Zygosporium sp. Mont. |
- |
- |
+ |
- |
25 |
O |
|
|
PNSM |
+ |
+ |
- |
- |
50 |
F |
|
|
WNSM |
|
+ |
+ |
- |
- |
50 |
F |
Table 6 Ecological impact of the species detected in three of the niches studied [indoor air (IA) from repositories, collected dust (CD) and documents surface (DS)]
*: Indicates that it is only valid for Aspergillus species. WNSM: White Non-sporulating Septated Mycelium. PNSM: Pigmented Non-sporulating Septated Mycelium. According to Esquivel et al.,32 when RF=100-81% the taxon is considered ecologically Abundant (A); 80-61% is Common (C); 60-41% is Frequent (F); 40-21% is Occasional (O); 20-0.01% as Rare (R).
The analysis of the QS indicates that different obtained values depended on the niches that were compared (IA indoor air, OA: outdoor air, CD: collected dust and DS: documents surface) and the detected genera (Table 7). When comparing IA with OA, the total QS(IA-OA) obtained varied from 0.8 to 0.9 depending on the analyzed sampling. As can be seen, the values are high, which indicates a high species similarity between these two niches, demonstrating the impact of the outdoor environment on the indoor environments of the archive, a result that is due to the existence of natural ventilation in the repositories. This behavior was previously demonstrated in a study conducted in various home environments.81 Furthermore, these findings agree with other earlier studies carried out in Cuban archives.9,13,49 Regarding the species belonging to the Aspergillus genus, the QS values were extremely high and ranged from 0.9 to 1.0, indicating that practically the same species were isolated in both types of environments. Cladosporium species had a similar behavior, i.e., the QS values varied from 0.7 to 1.0 depending on the analyzed sampling. However, for Penicillium species the QS value was lower (0.5), equivalent to moderate species similarity.
|
Relationship of taxa or species between: |
QSTOTAL |
QSAsp. |
QSClad. |
QSPen. |
|
IA-OA: Indoor air and outdoor air |
0.8 – 0.9§ |
0.9 – 1.0§ |
0.7 - 1.0§ |
0.5* |
|
IA-DS: Indoor air and documents surface |
0.4 §§ |
0.3 – 0.5§ |
0.6 - 0.7§ |
0.8* |
|
IA-CD: Indoor air and collected dust |
0.5 – 0.6 |
0.5 – 0.7§ |
0.8 - 0.9§ |
0.6* |
|
DS-CD: Documents surface and collected dust |
0.7 |
0.9 |
0.6 |
0.8 |
Table 7 Sørensen's similarity coefficients (QS) obtained by comparing the total number of taxa and the species belonging to the genera Aspergillus, Cladosporium and Penicillium isolated in all the studied niches
QSAsp: Refers the QS of the Aspergillus species. QSClad: Indicates the QS of the Cladosporium species. QSPen: Refers the QS of the Penicillium species. §: Indicates variations according to the sampling. §§: Signposts that the value was the same for the first and second sampling. *: Indicates that the similarity was only for the 1st isolation, since in the 2nd isolation Penicillium species were not detected in any of the studied environments.
The species similarity among IA and CD yielded total QS(IA-CD) that varied between 0.5 and 0.6, i.e., a moderate similarity. For the Aspergillus genus, the QS value ranged from 0.5 to 0.7, showing a moderate similarity, as for the species of Penicillium genus (QS=0.6). However, the similarity between Cladosporium species was high with QS values ranging between 0.8 and 0.9. This behavior also shows that the settled dust on indoor of the repositories directly influences the fungal diversity detected in the air of these places.
The comparison of the species isolated from the IA with those detected on the DS, gave a total QS(IA-DS) value of 0.4, representing a low similarity. QS values for Aspergillus species ranged from 0.3 to 0.5, showing low similarities. However, for the species of Cladosporium and Penicillium, QS values ranged from 0.6 to 0.8, respectively, showing moderate to high similarities. This indicates that the indoor air, although it can contribute to the contamination of the documents, is not the fundamental medium of this pollution.
The similarity of species detected on the DS and in the CD was high (QS(DS-CD)=0.7). But for Aspergillus and Penicillium species the similarities were even higher, with QS values ranging between 0.9 and 0.8, while for Cladosporium species the similarities were moderate (QS=0.6). This shows that the dust that settles on the documents is the main source of fungal contamination and that this could be aided by the air, hence the need to maintain systematic hygiene of the documents to prevent fungal growth on them. For this reason, the continuous implementation of the measures included in the Preventive Conservation Plan designed by the archive's curators is an imperative need to be able to preserve the documentary collections that they hoard, despite the fact that the building has serious constructive and humidity problems in its walls. However, the proposal to move the archive to a building with better constructive conditions continues to be a priority for the directive of this Cuban archive.
Overview of the influence of isolated fungal species on human health
Fungal spores and propagules are present everywhere, including on books, documents and it is not possible to eliminate them from the environment. Curators, conservators, and all workers in archives are therefore exposed to fungi on a daily basis during the working day,4,19 hence, systematic monitoring of environmental fungi, settled dust, and the surface of documents stored in archive repositories is important.
In this study it was possible to isolate fungal species that are considered toxigenic (A. flavus, A. ochraceus, A. parasiticus, A. terreus, A. versicolor, F. semitectum, P. brevicompactum, P. chrysogenum, etc.) and opportunistic pathogens (e.g. A. chevaliery, A. flavus, A. japonicus, A. niger, P. brevicompactum, P. chrysogenum, P. citrinum, etc.) from the indoor air of the repositories, from the collected dust and from the documents surfaces.39,82-85 Some of them, such as A. flavus, which is considered toxigenic and pathogenic,86,87 were detected in the indoor air of the repositories at concentrations greater than 50 CFU/m3, in dust at concentrations greater than 80 CFU/g, and on the surface of two (16.7%) of the analyzed documents. However, other species with the same pathogenic conditions86-88 were detected at lower concentrations (e.g. A. niger, A. oryzae and A. versicolor). Because the spores of many of the isolated Aspergillus and Penicillium species are small, they can be easily inhaled, particularly those smaller than 3mm, which can reach the alveoli causing pulmonary mycosis in immunosuppressed people (e.g. A. flavus, A. niger, P. citrinum, P. chrysogenum, etc.).9,59 Likewise, it has been reported that some of the species isolated in this study (A. flavus, A. oryzae, P. citrinum and P. janczewskii) are capable of growing at 37°C,9,34 a necessary and essential virulence factor for a species to be considered pathogenic,3,25 while others can excrete b-hemolysins and phospholipases9,34 standing out among them A. niger and A. flavus, the latter species classified as Biosafety Level (BL) 2 (BL-2).88
This study included the determination of fungal concentration and diversity in four niches of the Provincial Historical Archive of Villa Clara in Cuba (indoor air from repositories, outdoor air, collected dust on indoor environments, and documents surface) and related them to the environmental fungal quality of the repositories where documents are preserved. It was shown that in general the environmental quality of the repositories was good despite the existence of walls humidity in the building, but that natural ventilation played an important role in controlling the environmental fungal load, although some species were detected with concentrations higher than 50 CFU/m3, which is the concentration recommended as permissible according to regulations for other countries. The marked variability of the settleable dust loading was verified as a consequence of the construction conditions of the repositories, which undoubtedly influences the existing fungal concentrations not only in the dust itself but also in the indoor air of the repositories and on the documents surface. From all these studied niches, several fungal genera were isolated at different concentrations, with predominance of the Aspergillus, Cladosporium and Penicillium genera. Of them, a preponderance of the species A. flavus, Cl. cladosporioides, Cl. sphaerospermum was evidenced since they were isolated from the indoor air of the repositories, from the collected dust and from the documents surface.
The authors want to thank the funds given by the Ministry of Science, Technology and Environment (CITMA) of Cuba (grant number I-2118025001). We would also like to thank Dr. Matilde Anaya for the help provided in the statistical processing of data.
Authors declare that they have no conflict of interest.

©2022 Borrego, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.