Journal of
eISSN: 2373-437X


Research Article Volume 2 Issue 1
Advanced Laboratory Services, Rowan University, USA
Correspondence: Sheila Wood, Advanced Laboratory Services, 501 Elmwood Ave, Sharon Hill, 19079 Pennsylvania, USA , Tel 484 494 6125, Fax 484 494 6800
Received: November 06, 2014 | Published: January 2, 2015
Citation: Wood S, Datar A, Pabbat N, et al. Differentiation of Borrelia microbes from collagen debris and collagen fibrils in blood cultures. J Microbiol Exp. 2015;2(1):1-9. DOI: 10.15406/jmen.2015.02.00033
Lyme disease in humans is caused by Borrelia burgdorferi sensu lato, organisms that are known to attach to collagen in the body. The use of a matrix containing collagen as a part of the culture composition has given us the ability to cultivate Borrelia burgdorferi, B. afzelii, and B. garinii from the bloodstream using artificial media. The cultured Borrelia is identified by immunostaining using polyclonal and monoclonal antibodies. In order to distinctly differentiate the organisms from the collagen of this matrix that could be observed as background in the staining process, we developed an immunostaining procedure using polyclonal and monoclonal antibodies in combination with rhodamine fibronectin. The culture samples from both control organisms and patient samples were tested using the new immunostaining protocol. Results showed clear delineation of organisms compared to the collagen pieces gathered in the harvesting process. This new immunostaining process, used with in vitro cultivation, provides for precise identification of cultured organisms.
Keywords: collagen; immunostaining; Borrelia
ECM, extracellular matrix; GAGs, Glycosaminoglycans; BSK-II, Barbour Stoenner Kelly II; ALS, advanced laboratory services; RF, Rhodamine Fibronectin; PAb, Polyclonal antibody; Mab, Monoclonal antibody
Borrelia burgdorferi (B. burgdorferi) causes Lyme disease after being deposited in the skin by Ixodes ticks. A characteristic rash may appear and be accompanied by systemic symptoms such as fatigue, fever, headache, arthralgias or myalgias.1-4 In order to establish itself in the body and/or migrate to organs, the spirochete must interact with cells of the connective tissue and adhere to or colonize in the presence of macrophages, dendritic cells, fibroblasts, and the associated extracellular matrix (ECM).5,6 Some Borrelia surface proteins involved in the process of adherence to host ECM include a fibronectin receptor,7 proteins that bind to glycosaminoglycans (GAGs)8-10 and membrane lipoproteins that bind decorin.11-15 Multiple modes of interaction with the ECM help to explain the ability of the organism to survive, migrate, and thrive in many organs of the body.2,11,16 The ECM of the dermis is one of the first tissues encountered by Borrelia. The dermis is composed of protein and polysaccharide but is particularly rich in Type I collagen. The organism must make its way from the dermis into the bloodstream and from the blood into connective tissue of the organs. Two major candidates for adherence to and migration through the body include decorin and collagen.13,15,17-20 Studies have shown that Borrelia can bind to lattices prepared from acetic-acid extracted Type I collagen devoid of decorin and other proteoglycans. B. burgdorferi invaded and colonized these lattices.1
The interaction of Borrelia with collagen in the human body is recognized in the histopathological changes of skin conditions associated with Lyme borreliosis. Borrelia can break down substances within the ECM and can form micro colonies in collagen fibers.21,22-24 Another study showed that Plasmin-coated B. burgdorferi could enter and reside in tissues and that the spirochete preferred the interstitial ECM of colonized tissues.25 Tendons and ligaments are a structurally and topographically ideal retreat for Borrelia which may contribute to the chronic nature of the illness.21 Culturing Borrelia from human blood in vitro has proven to be challenging for the most astute scientists. Coulter et al.,26 cultured blood from suspected Lyme disease patients. They used the plasma from EDTA treated blood, which was inoculated into Barbour-Stoenner-Kelly II (BSK-II) media without antibiotics. Positive cultures were obtained from 22 of 82 patients. Wormser et al.,27 cultured plasma from untreated adult patients with erythema migrans. Of the 50 patients with blood cultures, 22 were positive. Sapi et al.,28 cultured serum from patients in modified BSK-H; 47 out of 50 were positive. Some factors that may influence the recovery of Borrelia from the blood could have to do with its ability to protect itself from the activity of complement and subsequently evade the actions of the immune system. Guner29 demonstrated that fibronectin protects spirochetes from complement-mediated lysis. He showed that both high and low passage B. burgdorferi 297 grown in BSK medium died and did not revive after 3 weeks incubation, perhaps due to lack of protection from complement. Grab et al.,30 showed that B. burgdorferi bound human plasma fibronectin and showed that fibronectin was present on the surface of these organisms. The investigators used immunofluorescence to show the presence of fibronectin bound to the surface of free swimming B. burgdorferi. The fibronectin appeared to cover the entire surface of the cell in a uniformly bound configuration. Their contention was that the uniformly bound fibronectin protected the Borrelia from complement in the circulation. These investigators also found (in preliminary experiments) that fibronectin enhanced the adhesion of B. burgdorferi to purified human collagen. Also, Borrelia has been shown to bind collagen directly, specifically by the adhesins Csp A and Csp Z. Borrelia adhesins that bind fibronectin include BBK32, Rev A, Rev B, BBO347, Csp A, and Csp Z.31 Fibronectin-collagen interactions are well documented and play key roles in cellular processes, such as adhesion, migration, growth, and differentiation. Fibronectin is a high molecular weight multi-domain protein composed of three module types. Recent studies performed on fibronectin-collagen interactions show a cooperatively of binding and multidomain units in fibronectin which are all involved in binding to collagen. It was shown that fibronectin has six collagen-binding modules that interact cooperatively with a single collagen site. Collagen I possesses four equipotent sites for fibronectin binding.32
Although reports show that Borrelia produces adhesins that bind fibronectin, it is clear that this production can vary based on the formulation of the media used to grow the organism. The production of the adhesin BBK32 by Borrelia spirochetes may serve to tether it to fibronectin. Moriarty et al.,33 described the interactions of Borrelia with fibronectin when it was grown BSK II medium. BSK II medium contains gelatin (amounts may vary per laboratory) whereas BSK-H does not contain gelatin. Borrelia grown in BSK II medium was used in the mouse model to study their interactions with mouse vascular epithelium. The interactions in the mouse blood were described as a transient “tethering” of BBK32 to fibronectin, in a strong yet brief attachment, occurring anywhere along the length of the Borrelia with very rapid association and dissociation. The second interaction, perhaps the most important to pathogenesis was the attachment of the organism to GAGs in a less strong interaction that had a slower association and dissociation rate. Both processes preceded migration across the mouse vascular epithelium. In this experiment, Borrelia was grown in BSK II media which differs from the modified BSK-H media used in our laboratory. This experiment shows that BBK32 is present on the organism when it is grown in BSK II and when it is in contact with mouse blood in vivo. Other investigators34 have shown that in the isolates of B. burgdorferi that do not produce BBK32 during in vitro culture,35 genetic manipulation with a shuttle vector resulted in production of BBK32. The organism could then bind to GAGs on the surface of mammalian cells and to fibronectin in a micro titer plate. This report says that BBK32 is expressed by spirochetes found in mice but not by Borrelia grown in culture using BSK II at 33 C. Also, this fact was confirmed by Rathinavelu and de Silva, who used RT-PCR and compared levels of BBK32 mRNA produced by spirochetes within feeding ticks versus in BSK II culture media.36 Li et al.,37 showed that B. burgdorferi lacking BBK32 retained full pathogenic capability in mice. BBK32 was expressed at different phases of the Borrelia life cycle and was primarily expressed in mice and engorged ticks. BBK32 expression varied over a wide range and there was nearly a 1000-fold increase from the lowest level in ticks to the highest level in mouse tissues. Although there was a 2.8-fold increase in BBK32 expression in vitro when the spirochetes were switched from 25°C to 37°C, this difference could not explain the difference encountered in feeding ticks. Even the highest increase in feeding ticks was still 6-29-fold lower than in mouse tissues.
Yang et al.,38 showed a difference in genetic regulation when organisms were grown in BSK II versus BSK-H. For example, the authors showed that organisms grown in BSK-H were more motile and actively replicated, compared to those grown in BSK II. Both pH and temperature were shown to affect expression of genes differently in the two different types of media. They concluded that BSK-H is superior to BSK-II for studies in gene regulation. Advanced Laboratory Services (ALS) uses a blood culture medium modified BSK-H that contains 12% rabbit serum and a collagen matrix as described by.28 The culture media is inoculated with the patient’s blood and both short term (6 days) and long term cultures (8 to 16 weeks) are set up. The 8 week to 16 week culture contains the collagen matrix. The use of this matrix enhances the growth of Borrelia in this artificial laboratory culture medium. The substrate is composed of Rat Tail Collagen Type I with rat tail tendon as the source of the collagen (Cat no. 354557, Corning).39 The cultures are incubated for up to 16 weeks, and are harvested at intervals of 8 weeks and 16 weeks. Organisms attached to and/or growing in the collagen are harvested by scraping the collagen off the slide. Therefore, it is to be expected that some collagen could be visible in the background of stained slides. Collagen shed from the coated slide showed cross-reactivity with the polyclonal and the monoclonal antibody used in the laboratory. Therefore, our goal became to find a substance that bound collagen and generated a background signal at a different wavelength than our labeled antibodies. For this purpose, we used rhodamine fibronectin (RF) from bovine plasma (Cat no. FNR01, Cytoskeleton, Inc.).40 This product has been shown to bind integrins, and ECM components including collagen, fibrin, and heparin sulfate proteoglycans. The fibronectin is modified to contain covalently linked rhodamine at random surface lysines. The positive control used in these experiments was B. burgdorferi B31 ATCC 35210, originally isolated from a tick. Successive passages of control organisms in the laboratory can result in loss of plasmids, chromosomal rearrangement, variation in protein expression and the lack of production or utility of flagella, resulting in non-spiral forms.41 Labandeira-Rey & Skare42 showed that decreased infectivity of B. burgdorferi B31 was related to the lack of linear plasmids 25 and 28-1. The morphology of this control organism is clearly different from that of virulent Borrelia grown from human blood.43,44 Purser and Norris also evaluated plasmids from B. burgdorferi B31 and showed plasmid correlation to infectivity.35 For example, in each of 19 clones, when plasmid Ip28 was absent, the strain was not infective. Infectivity has been correlated to motility, and hence periplasmic flagella, which convey the spiral shape. Also, both adhesins and invasion factors are carried on plasmids and may be lost by the organism.41,45,46
Verification that antibodies to Borrelia react with collagen
To verify that the antibodies used for Borrelia testing cross react with collagen, a Coplin jar was set up mimicking patient specimen long-term culture method with the exception that no organisms were added to the Coplin jar. Ten mL of the media from the Coplin jar was collected (collagen coated slides in the Coplin jar were scraped) and immunochemistry was performed as per current testing methods.28
Culture of established cell lines and patient samples
B. burgdorferi B31 (Bb B31) (ATCC # 35210) was obtained from American Type Culture Collection. It was maintained in the lab for use as a positive control strain and used in these experiments at passage number five. Bb B31 was maintained in BSK-H media (Cat no. B3528, Sigma-Aldrich) in 5% CO2 and 95% humidity. In addition, B. burgdorferi S297 was added as a second control (fibronectin and rabbit serum controls) and maintained in conditions identical to Bb B31. It was also used at passage number five. Escherichia coli (E. coli) ATCC 25922 was used as a negative control and maintained in standard Luria Broth media (Sigma) at 37°C. These were the control organisms used in the study. Patient samples used in this study were cultured as previously described in the 2013 article by Sapi et al.28 published in the International Journal of Medical Sciences. A series of positive organisms from patient samples that show pleomorphic configurations are shown to compare and contrast control B. burgdorferi B31. Patient samples that were in long-term culture were included in the initial study. Fifty-five samples were observed to ensure efficacy of the double stain. The procedure was implemented and to date 1,285 patient samples have been tested using the double stain.
Immunochemistry
For all the experiments, 100µl of cell suspension (control organisms) from culture were harvested, placed in 1X PBS (Cat no. BP2944, Thermo Fisher Scientific). Ten mL of the patient samples were collected from the long-term cultures and all samples were centrifuged at 9,600 xg, 10 min. The supernatant was discarded and pellets were resuspended in 75µL of 1X PBS and smeared on super frost plus slides (Cat no. 12-550-15, Thermo Fisher Scientific). The slides were allowed to air dry for 90 min, and then incubated at 37°C for 20 min. For polyclonal antibody (PAb) FITC labeled Borrelia specific antibody (Cat no. PA-1-73005, Pierce) staining procedure, the slides were fixed in ice cold acetone (Cat no. A-11-4, Thermo Fisher Scientific) for 10 min at -20°C, air dried and incubated for another 20min at 37°C. For monoclonal antibody (MAb) B. burgdorferi specific antibody (Cat no. PA-1-73006, Pierce) staining procedure, the slides were fixed in 4% paraformaldehyde (Cat no. 158129, Sigma) prepared in 1X PBS at room temperature for 20 min, followed by ice cold methanol (Cat no. A433P-4, Thermo Fisher Scientific) fixation for 20 min at -20°C, and 0.3% Triton X-100 prepared in 1X PBS at 37°C for 20 min. The slides were washed with 1X PBS and water, dried and blocked with normal goat serum (Cat no. NC9270494, Thermo Fisher Scientific) for 20 min at room temperature, washed again with 1X PBS and water and air dried. Both antibodies (PAb and MAb) were diluted in 1% BSA (Cat no. A4503, Thermo Fisher Scientific) made in 1X PBS. PAb was used at 1:50 dilution (final concentration of PAb used was 90µg/mL) and MAb was used at 1:10 dilution (final concentration of MAb used was 10µg/mL).
Testing rhodamine fibronectin
The first objective was to check if RF (Cat no. FNR01, Cytoskeleton, Inc.) Cross-reacts and binds to the control organisms when used with PAb. One mg/mL stock of RF was prepared and 1:10, 1:50, 1:100, 1:500, and 1:1000 dilutions of RF were tested with 1:50 dilution of PAb via two conditions.
Reading the slides
A fluorescent microscope affixed with a camera was used to view all slides and a computer-generated software program assisted in the production of photographs. The microscope had the capability to read FITC (494 nm ex/518 nm em), DAPI (358 nm ex/461 nm em), DyLight488 (488 nm em) and rhodamine (543 nm ex/576 nm em). All images were taken at 400x magnification. Collagens versus organism pictures were taken on the same frame with a change in filter. The entire portion of a 20 x 30 mm area on a slide was prepared and read for each patient. In order to be photographed, an object had to have the appearance of a rod shaped, spiral or partially spiral organism. Varying degrees of spiral structures are seen with Borrelia and often, the organisms appear in clusters or clumps. Our requirement for delineation of Borrelia is a clearly defined microbial structure, microbial structure with spiral curvature, and/or microbial structure with clumping and spiral curvature (examples of Borrelia morphology are illustrated in figures that appear in the Results section). Any time a FITC stained object was photographed, the same frame was photographed using the rhodamine filter. Objects giving a bright orange glow were considered positive by RF and not organisms. Slides were reviewed by the laboratory supervisor prior to release.
Fibronectin and rabbit serum controls
Controls were established to determine if fibronectin or components in rabbit serum were possibly coating the organisms as they grew in culture. A fibronectin coating could perhaps enhance the binding of organisms to the collagen matrix and/or prevent binding of RF to the organisms. B. burgdorferi B31 and B. burgdorferi 297 were grown in BSK-H with 6% partially hemolyzed rabbit serum, and modified BSK-H with 12% partially hemolyzed rabbit serum.28 The collagen slide was omitted from each BSK-H set up. The organisms were harvested and centrifuged at 9,600 g for 10 min. The pellets were reconstituted in 1) PBS; 2) PBS containing 1 µg/mL human fibronectin; 3) PBS plus 12% partially hemolyzed rabbit serum. The tubes were incubated at 34o C for 30 min. A no organism control was performed for the PBS-fibronectin and the PBS 12% rabbit serum conditions. Each slide was stained with PAb-RF double stain.
Results of monoclonal and polyclonal reaction with collagen–no organism control
The results revealed that both polyclonal and monoclonal antibodies cross-react with collagen. Although these shapes are not clear spirochetes, it became important for us to identify these pieces of collagen as background (Figure 1).
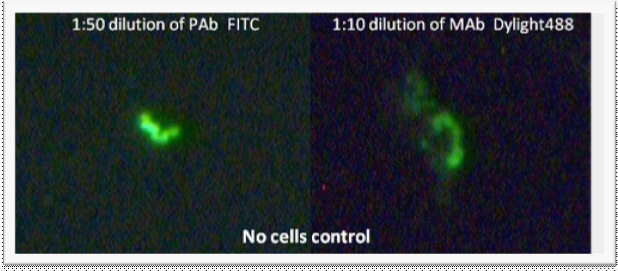
Figure 1 Immunochemistry (with polyclonal (PAb) and monoclonal (MAb) antibodies) performed on media taken from Coplin jar containing collagen coated slides. No organisms were added to the Coplin jar. Images were taken at 400x magnification.
Results of control organisms and polyclonal antibody plus RF
RF showed no cross-reactivity with the control organisms when tested with the polyclonal antibody to B. burgdorferi (Figure 2).
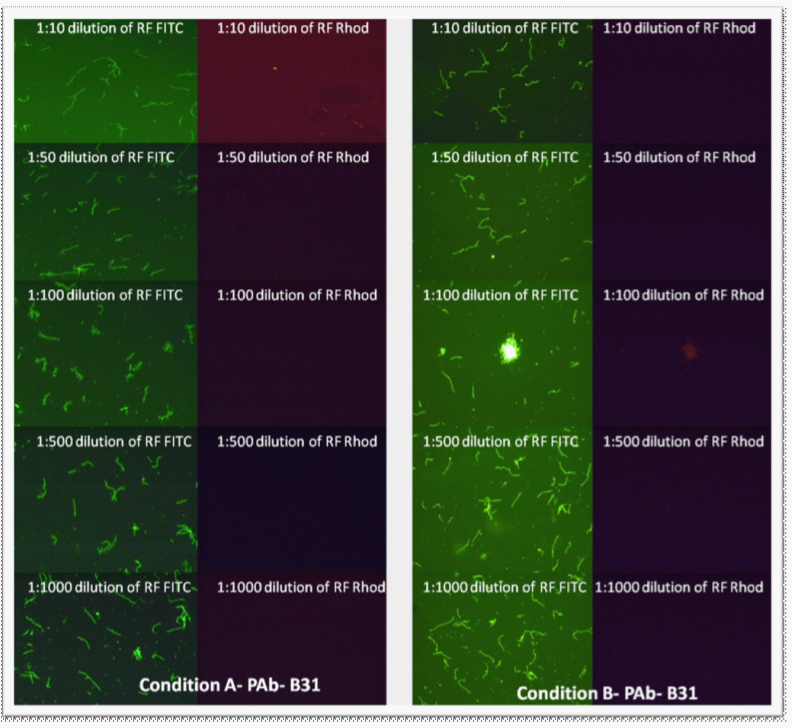
Figure 2 Rhodamine fibronectin tested at different dilutions (made from 1 mg/mL RF stock) with polyclonal antibody (1:50 dilution) on B. burgdorferi B31 and E. coli with Conditions “A” and “B.” E. coli did not stain; therefore the pictures are not included. Images were taken at 400x magnification.
Results of control organisms and monoclonal antibody plus RF
Since 1:50 and 1:100 dilutions of RF worked well with PAb (1:50), these dilutions were chosen for further experiments. Although both Conditions “A” and “B” were acceptable, “Condition B” was chosen for further testing for convenience. For the testing of RF with MAb, 1:50 and 1:100 dilutions of RF were tested in combination with MAb, experimental “Condition C.” With MAb, a spotty background was observed with RF at both 1:50 and 1:100 dilutions (Figure 3). Further experiments used a 1:25 dilution of RF.
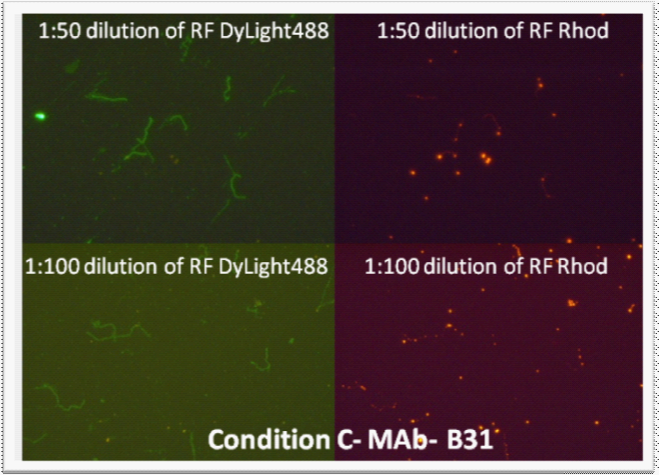
Figure 3 Rhodamine-Fibronectin tested at 1:50 and 1:100 dilutions (made from 1 mg/mL RF stock) with monoclonal antibody (1:10 dilution) on B. burgdorferi B31 and E. coli with “Condition C”. E. coli did not stain; therefore the pictures are not included. Images were taken at 400x magnification.
Results of control organism grown in culture medium containing collagen coated slides stained with monoclonal and polyclonal antibody plus RF. Patient sample results shown for comparison
Control organisms were then grown in culture media containing collagen coated slides. Harvesting was performed as per patient cultures (collagen slides were scraped). RF was incorporated into the monoclonal and polyclonal staining at a 1:25 dilution. The slides showed well presenting organisms when stained with PAb-FITC and MAb Dylite and little background was seen with the RF (Figure 4).
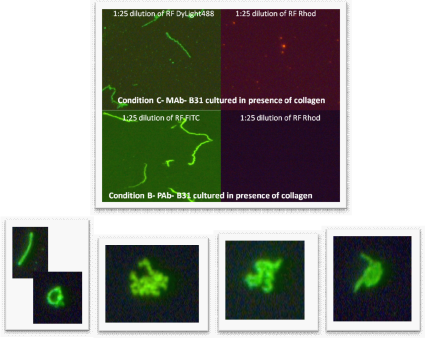
Figure 4 Top four pictures - Rhodamine-Fibronectin tested at 1:25 dilution (made from 1 mg/mL RF stock) with monoclonal antibody (1:10 dilution, “Condition C”) and polyclonal antibody (1:50 dilution, “Condition B”) on B. burgdorferi B31 and E. coli. E. coli did not stain; therefore the pictures are not included. Images were taken at 400x magnification.
The lower four pictures left to right are all from different patient blood cultures stained with the double stain. 1) One long form and one round form that is changing from spheroplast form (Gemma) to long form. 2) A cluster of spirochetes indicating the beginning of biofilm formation. 3) Two to three spirochetes with the spiral morphology that is distinct. 4) A spirochete emerging from a Gemma or spheroplast.
Results of some patient samples in complete medium with collagen-coated slides using polyclonal antibody plus RF
After establishing the conditions for using RF with both PAb and MAb by testing on control organisms in culture, the next step was to test the stain for background in patient samples. Figures 5-7 provide some examples of the ability of the RF stain to distinguish background collagen. In (Figure 5), the frames appear to be collagen debris. Figure 6 shows an upper and lower portion of the material in each frame. In the first frame, the upper portion, represented by shadow, is attached to the organism which stains brightly with FITC. In the lower frame, the collagen stains brightly with RF and the organism does not stain with RF. Figure 7 has distinct monomers, reminiscent of human collagen.47
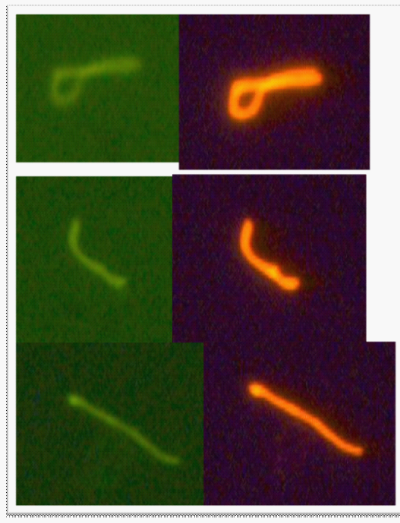
Figure 5 This photograph shows three particles from a patient sample that stain with RF. The green portion is Ab-FITC and the orange is RF.
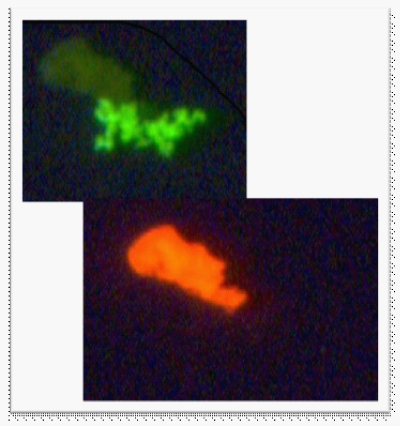
Figure 6 In the top frame, half of the material stains with Borrelia PAb-FITC. In the bottom frame, the other half of the material stains brightly with RF.

Figure 7 In this RF staining material, monomers can be seen, reminiscent of a human collagen fibril (Gelman et al.,47).
Results of continued use of monoclonal and polyclonal staining plus RF on patient samples
Table 1 shows the number of slides that have been processed in our lab using the double stain. We have used this procedure on 1285 culture slides and 28 of these slides showed extraneous material from the collagen matrix. The double stain helped elucidate and confirm the presence or absence of Borrelia on the slide. Some views clearly showed Borrelia attached to collagen scraped from the collagen-coated slide. Our ability to clarify organism from collagen has been improved by two percentage points.
|
Number of patient samples viewed using double stain from all samples tested both positive and negative |
1,285 |
|
Number of these slides that used RF for determination of positivity of negativity |
28 |
|
Percent in which RF was useful for determination of positivity or negativity |
2% |
Table 1 The percentage of RF frames used to determine validity from all samples tested, both positive and negative
Results of control slides from Borrelia grown in different BSK-H formulations and exposed to fibronectin or 12% serum
Control slides with B. burgdorferi B31 and B. burgdorferi 297 that were grown in either BSK-H with 6% serum or BSK-H with 12% serum, harvested, and incubated with human fibronectin or 12% serum, showed no difference in staining when the double stain was used.
From the time that the Borrelia organism is deposited in the skin by a tick, it travels throughout the body via attachment to molecules in the tissue and a series of immune manifestations occur in the body. As the organism migrates into the bloodstream, out of the bloodstream, toward organs and into organs, symptoms occur depending on how the immune system responds. During this journey, clear binding targets include components of the interstitial ECM. Symptoms can vary in individual patients enough to confuse the clinical picture. Detection of the organism from the bloodstream of infected individuals has been attempted and studied since the 1990’s.26,27 Borrelia has been shown to attach to collagen in vitro under the right conditions and to intermittently serve as a tether to fibronectin in the mouse model.33 In our laboratory we have witnessed the ability of commercial polyclonal and monoclonal antibodies to attach to Type I collagen from our modified laboratory media. One possible explanation for this cross-reactivity with collagen is that since Borrelia is known to attach to collagen in vivo and to evade the immune system in vivo16 it may contain outer surface structures that are similar enough to collagen to elicit the production of collagen interactive antibodies.
Another opportunity for cross reactivity with components found in the bloodstream exists. Since the antibody is made against whole organism grown in the laboratory, the opportunity for the organism to be coated with either fibronectin or a GAG, such as chondroitin sulfate, exists. The growth medium contains serum which may be a source of fibronectin and/or GAGs. The observation of fibronectin binding to and protecting Borrelia from complement in vivo was shown by.29 Coverage of the organism by fibronectin may also be taking place in the laboratory culture medium. If organisms coated with fibronectin (from growth in culture) are used in the antibody generation process, cross-reacting epitopes could result. Such epitopes could be reactive with fibronectin and/or GAGs that naturally bind the outer surface of the organism.
Clearly presented data from various investigators has shown lack of production of fibronectin-binding adhesin BBK32 on the one hand34 and production of BBK32 adhesin38 on the other hand by Borrelia. Results correspond to different growth environments found in BSK II medium, BSK-H medium, the bloodstream if mice, or other artificial environments. The resulting absence or presence of adhesins to fibronectin could influence the organisms’ ability to bind to RF. Our results, including the separate set of controls that were performed, point to the fact that Borrelia grown in our culture conditions does not impart to the organism an available adhesin with the capacity to bind RF. However, collagen debris from the matrix in the modified BSK-H media binds to RF with resultant fluorescence (Figure 6).
Another factor that could contribute to RF binding to collagen and not to fibronectin in our culture and stain conditions, could be the construction of the RF molecule. Fibronectin is modified to contain covalently linked rhodamines at random surface lysines. Binding to fibronectin could be sterically inhibited. Investigators have shown that Borrelia has the capacity to bind fibronectin in its environment and remain fully active, free swimming Borrelia.30 It is conceivable that the organisms either come from the bloodstream covered in fibronectin or acquire a covering of fibronectin in culture that allows them to perhaps survive the effects of complement and attach more effectively to collagen. It is clear that if either of these is a possibility, the organism reacts with PAb and MAb but does bind to RF with resultant fluorescence. Another suggestion would be that the binding of antibody to antigens on the organism have higher affinity of binding than the fibronectin in the RF molecule. The question remains, if the organisms acquire a coating, or partial coating, of fibronectin, either in culture or in the bloodstream, do they attract antibodies that also bind to a similar antigen. Evidence of Borrelia making changes to its outer antigens to evade the immune system may involve the acquisition or production of a fibronectin-like and/or a collagen-like antigen capable of cross reacting with specific antibody.
Our development of a double stain that reacts with Borrelia organisms and also with contaminating background collagen enables our laboratory to eliminate a 2% risk of misinterpretation of our slides. RF staining, in combination with the polyclonal and monoclonal staining, helped in the delineation of collagen present as background in stains from blood culture. Often in the development of detection methods for microbes, it becomes necessary to take advantage of the organism’s mechanism of action during the pathogenesis process. Borrelia has been shown to inhabit the ECM in the body and has been shown to attach to collagen. Collagen is not degraded solely by the organism. Therefore, it becomes a clear advantage to use this substance when growing the organism in culture. As part of the cultivation and staining process, however, care must be taken to ensure the exacting nature of the stains by providing a means to counter any interference from background materials. Of the 1,285 patient slides read to date using double staining, approximately two percent proved to be collagen instead of bacteria. This addition to our methods clearly provides an advantage when reading the slides.
|
BSK-H |
BSK II (CMRL)* |
||
|
Ingredient |
mg/L |
Ingredient |
mg/L |
|
Arginine |
57 |
Arginine |
70 |
|
Citric acid (tetrasodium) |
700 |
Sodium citrate |
800 |
|
2'-Deoxycytidine-HCL |
11.6 |
2'-Deoxycytidine |
10 |
|
Flavin adenine dinucleotide |
0.106 |
Flavin adenine dinucleotide |
1 |
|
Gelatin |
0 |
Gelatin |
14000 |
|
Magnesium Sulfate |
97.69 |
Magnesium Sulfate |
200 |
|
Sodium Acetate |
50 |
Sodium Acetate |
83 |
|
Sodium Glucaronate |
3.88 |
Sodium Glucaronate |
4.2 |
|
Sodium Phosphate monobasic |
122 |
Sodium Phosphate monobasic |
140 |
Table 2 BSK II and BSK-H media
*Composition varies by laboratory.
The authors would like to thank Joseph Burrascano, MD, Phillip Tierno, PhD, and Eva Sap, PhD for their review and comments. We would also like to thank Lauren Burawski, MA for her assistance in preparing this manuscript.
Authors declare that there is no conflict of interest.

©2015 Wood, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.