Journal of
eISSN: 2373-437X


Research Article Volume 1 Issue 4
1Department of Epidemiology, Fielding School of Public Health, UCLA (University of California, Los Angeles), USA
2David Geffen UCLA School of Medicine, USA
3Fundacao Oswaldo Cruz (FIOCRUZ), Brazil
4Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, USA
5Hospital Geral de Nova Iguacu, Brazil
6Hospital Federal dos Servidores do Estado, Brazil
Correspondence: Claire C. Bristow, Department of Epidemiology, Fielding School of Public Health, UCLA (University of California, Los Angeles), Los Angeles, CA 90095, USA
Received: July 05, 2014 | Published: August 11, 2014
Citation: Bristow CC, Adachi K, Saines KN, et al. Characteristics of the sample adequacy control (SAC) in the cepheid Xpert® CT/NG assay in female urine specimens. J Microbiol Exp. 2014;1(4):148-151. DOI: 10.15406/jmen.2014.01.00026
Background: The Xpert® CT/NG (Cepheid Sunnyvale, CA) is a rapid, fully automated real-time polymerase chain reaction test that simultaneously detects Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG). It has high sensitivity and specificity, but also includes a Specimen Adequacy Control (SAC). SAC controls for false negative results by confirming adequate patient sample and appropriate testing conditions. SAC is quantified by its cycle threshold (Ct), the number of cycles required to detect the presence of a single copy human gene. A lower SAC indicates an earlier Ct and more human cellular material detected. Our objectives were to describe the frequency and distribution of SAC Ct values and observe any correlations with detected infections.
Methods: Urine samples from 1382 HIV-1-infected pregnant women, collected at the time of labor/delivery underwent Xpert® CT/NG testing. Mean SAC Ct values and standard deviation (SD) were calculated. Student’s t-test was used to compare mean SAC Ct values to a reference of urine samples negative for CT and NG.
Results: The urine CT positivity was 17.9% (248/1382) and NG, 4.6% (63/1382). The mean SAC Ct value in urine from women without CT or NG was 28.09 (SD: 4.12) and higher than the mean SAC Ct value for CT positive specimens (27.29, SD: 3.84(P=.0054)), NG positive specimens (26.23, SD: 3.09(P<.0001)), specimens positive for both CT and NG (26.41, SD: 3.01(P=.0027)).
Conclusion: Lower SAC Ct values were significantly associated with chlamydial and gonococcal infections. Further studies should be conducted to determine the utility of SAC Ct values for identifying the presence of increased human cellular material and infection.
Keywords: sample adequacy control, chlamydia, gonorrhea, diagnosis, inflammation
HIV, human immunodeficiency virus; ART, anti-retroviral therapy; NICHD, national institute of child health and human development; CDC, center for disease control; HPTN, HIV prevention trial network; STI, sexually transmitted infection; CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae; Ct, cycle threshold; SAC, specimen adequacy control; SD, standard deviation; PCR, polymerase chain reaction; HMBS, hydroxymethylbilane synthase; SPC, sample processing control; PCC, probe check control; FDA, food and drug administration.
Xpert® CT/NG, a rapid automated real-time PCR assay, includes a Specimen Adequacy Control (SAC). A secondary use of the SAC cycle threshold values may be as a marker for inflammation.
Sexually transmitted infections (STIs) of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) continue to place an immense health burden on women worldwide. CT and NG infections are the most common STIs and accounted for 211.8 million cases globally in 2008.1 In the United States in 2009 alone, over 1.2 million chlamydia infections were reported to the U.S. Center for Disease Control (CDC) making it the most commonly reported notifiable disease.2,3 Gonorrhea is the second most commonly reported notifiable disease in the United States with over 300,000 cases reported in 2009.
The immunologic response triggered by lower genital tract infections with Chlamydia trachomatis and Neisseria gonorrhoeae leads to significant inflammation of the cervico-endometrial tissue.4 Due to infection and the associated inflammatory response, several important sequelae may result from these conditions, including pelvic inflammatory disease, ectopic pregnancy, and infertility.3‒6 Routine screening of STIs is recommended for young women and at-risk groups including pregnant women because of the frequency of infection and the adverse outcomes associated with untreated infections.
The Cepheid Xpert® CT/NG (Cepheid Sunnyvale, CA) is a point-of-care fully automated real-time polymerase chain reaction (PCR) test. It is a rapid molecular assay for simultaneous detection and differentiation of DNA from N. gonorrhoeae and C. trachomatis. The assay is contained in a cartridge that is run on the GeneXpert platform, which can be used with cartridges aimed at diagnosis of other infections. Xpert® CT/NG assay, which has received clearance by the Food and Drug Administration (FDA), has both high sensitivity and specificity.7 The Xpert® CT/NG assay amplifies one unique chromosomal gene target (CT1) for the detection of C. trachomatis, and two unique chromosomal gene targets (NG2 and NG4) for detection of N. gonorrhoeae. Both NG targets need to be positive for the Xpert® CT/NG assay to return a positive NG result. The amplification of those targets is indicated by a pathogen-specific cycle threshold (Ct) value for each target. The pathogen-specific Ct values in the GeneXpert platform have been explored as a measure of bacterial load in some of the other assays manufactured by GeneXpert, including that for MRSA and tuberculosis.8‒10
A failure detection mode included in the assay is the Sample Adequacy Control (SAC), which targets a single copy human gene that should be present in each specimen. The SAC controls for false negative results where no human cells are present by confirming adequate patient sample has been collected and appropriate testing conditions have occurred. In the Xpert® CT/NG assay, the SAC is quantified by its Ct, the number of cycles required to detect the presence of 1 human gene target, hydroxymethylbilane synthase (HMBS). A lower SAC Ct value indicates an earlier cycle detection threshold and more human cellular target in the specimen. Our objective was to describe the frequency and distribution of SAC cycle threshold values and observe any correlations with detected infections.
We performed a secondary analysis of data collected as part of the 040 study.11 The study enrolled mothers and infants from Brazil, South Africa, Argentina and the United States to evaluate the efficacy of three different antiretroviral regimens to neonates in the prevention of intrapartum mother-to-child transmission of Human Immunodeficiency Virus (HIV).
Patient population
The study population in this analysis consisted of HIV-1-infected pregnant women, who had not received antiretroviral treatment before the onset labor because of late presentation for medical care. Mothers and infants were enrolled in the 040 parent study within 48 hours of the infant’s birth. Infants were followed for the first 6 months of life to determine whether mother-to-child transmission of HIV occurred.
Specimen collection
Stored maternal urine samples collected at the time of labor/delivery or within 48 hours of giving birth were tested for the presence of CT and NG using the Xpert® CT/NG assay. The assay was performed on the Cepheid GeneXpert Instrument System which automate and integrate sample purification, nucleic acid amplification, and detection of the target sequences in samples using real-time PCR assays. Single-use disposable cartridges that hold the PCR reagents and host the PCR process were used.
Each self-contained cartridge controls for failure modes through: Sample Processing Control (SPC), a Sample Adequacy Control (SAC), and a Probe Check Control (PCC), included in the cartridge. The SPC is present to control for adequate recovery and amplification of the target bacteria using non-pathogen Bacillus globigii DNA. The SPC verifies that binding and elution of target DNA have occurred if the target organisms are not found to be present in the sample. The SAC reagents detect the presence of the gene encoding HMBS, a single-copy human cellular house-keeping gene, to monitor whether the sample contains human DNA. A negative SAC indicates that inadequate numbers of human cells are present in the sample due to sample degradation, insufficient mixing, or because of an inadequately collected specimen. The PCC verifies reagent rehydration, PCR tube filling in the cartridge, probe integrity, and dye stability.
Aliquots (7 mL each) of stored frozen urine from HIV-1-infected mothers were thawed and transferred to a special collection tube and shipped on ice packs for next day delivery to the testing laboratory for evaluation. Xpert® CT/NG results were reported as positive, negative or indeterminate (reading invalid, error or no result). Indeterminate test results were repeated up to twice. Each result included a SAC Ct value. In each PCR cycle the target material, HMBS, is doubled. Therefore, a difference in a few number of cycles a substantial difference in the absolute amount of target material present. HIV infection in infants was determined by HIV DNA PCR (Roche Molecular System, Indianapolis, IN) during the conduct of the primary study. Infants with a positive HIV-1 DNA PCR assay underwent repeat testing as soon as possible. Confirmed HIV-1 infection was defined as two positive results from specimens collected on different days. The primary study endpoint for the 040 parent study was HIV-1 infection at 3 months of age. None of the infants enrolled in 040 were breastfed.
Data analysis
Mean SAC Ct values and standard deviation (SD) were calculated for each result category. Student’s t-test was used to compare SAC Ct mean values for infection categories to the SAC Ct mean values for urine specimens that were negative for both CT and NG. Where variances for two comparison groups were significantly different using the folded F-statistic, the Satterthwaite variance estimator was used for the t-test. Where there was no evidence for a difference in variances, the pooled variance estimator was used. Analyses were conducted using SAS v9.3 (Cary, NC).
Ethical approval
The UCLA Medical IRB approved the parent clinical trial study and this sub-study. Inclusion in the study was only granted for those women that could provide informed consent. Written informed consent was obtained from all participants in the study which included consent on behalf of the infants from their mothers. The UCLA IRB number is IRB#13-000079 and the study was approved on 3/6/2013 and IRB approval was renewed on 2/3/2014.
Urine samples from 1406 HIV-1-infected women were tested using the Xpert® CT/NG assay on the GeneXpert platform. Of the 1406 specimens, 47 (3.3%) had indeterminate results. Those samples were rerun up to two times, and 23 of the 47 samples retested gave a valid result. The 24 (1.7%) samples that continued to give indeterminate results were excluded from analysis. Amongst the 1382 samples remaining after exclusion of invalid/error results, results were positive for CT in 248 (17.9%). NG infection was detected in 63 (4.6%) of the samples. Thirty-five (2.5%) of specimens were positive for with both CT and NG.
The mean SAC Ct value in urine from women not infected with CT or NG was 28.09 (SD: 4.12), this was used as the reference for bivariate comparisons. This reference cycle threshold value was higher than the mean SAC Ct value for CT positive specimens, NG positive specimens and those co-infected with both CT and NG. The SAC Ct value of specimens from women infected with CT was 27.29 (SD: 3.84, P=0.0054). For specimens from women infected with NG, the SAC Ct value was 26.23 (SD: 3.09, P<0.0001). Amongst the specimens from women co-infected with both CT and NG, the SAC Ct value was 26.41 (SD: 3.01, P=0.0027) (Table 1, Figure 1).
|
N |
Mean |
Std |
Median |
IQR |
Max |
Min |
p-value |
CT+ |
248 |
27.29 |
3.84 |
27.05 |
4.40 |
38.90 |
17.70 |
0.0054 |
NG+ |
63 |
26.23 |
3.09 |
26.20 |
3.60 |
32.10 |
17.70 |
<.0001 |
Co-infected, NG+ and CT+ |
35 |
26.41 |
3.01 |
25.90 |
3.90 |
32.10 |
17.70 |
0.0027 |
At least one infection, NG+ or CT+ |
276 |
27.16 |
3.80 |
27.05 |
4.35 |
38.90 |
17.70 |
0.0007 |
Uninfected with CT and NG |
1106 |
28.09 |
4.12 |
27.6 |
5.60 |
43.40 |
18.70 |
REF |
TOTAL |
1382 |
27.90 |
4.07 |
27.50 |
5.40 |
43.40 |
17.70 |
|
Table 1 Sample adequacy control (SAC) cycle threshold (Ct) values for urine samples from HIV-1-infected pregnant women by infection status.
P-values were generated using student’s t-test comparing SAC Ct mean values for infection categories to the SAC Ct reference shown.
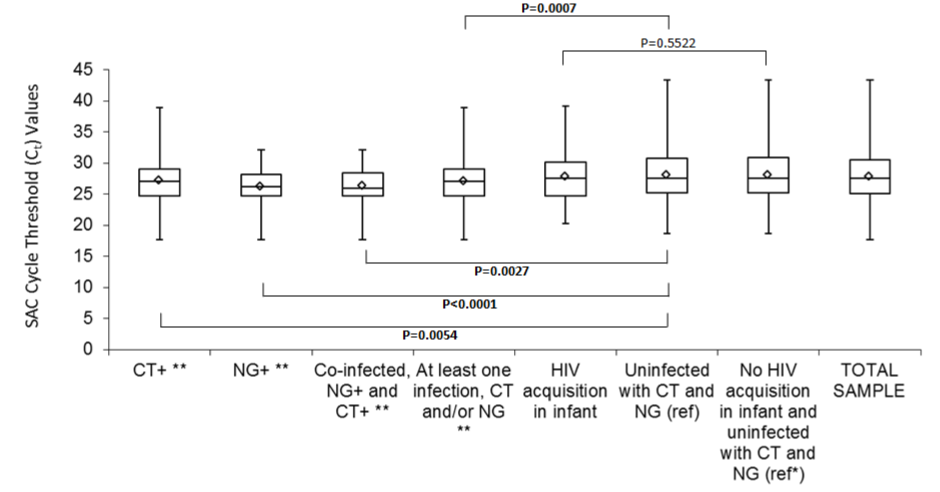
Specimens from mothers who transmitted HIV infection to their infants had a mean SAC Ct value of 27.81 (SD: 4.06). This value was not significantly different from that of specimens obtained from women uninfected with NG and CT who did not transmit HIV to their infants, mean SAC Ct value of 28.11 (SD: 4.12, p= 0.4517) (Table 2).
|
N |
Mean |
Std |
Median |
IQR |
Max |
Min |
p-value |
HIV acquisition in infant |
119 |
27.81 |
4.06 |
27.60 |
5.50 |
39.10 |
20.30 |
0.5522 |
No HIV acquisition in infant and uninfected with CT and NG |
1015 |
28.11 |
4.12 |
27.60 |
5.70 |
43.40 |
18.70 |
REF* |
Table 2 Sample adequacy control (SAC) cycle threshold (Ct) values for urine samples from HIV-1-infected pregnant women by infection status
P-values were generated using student’s t-test comparing SAC Ct mean values for infection categories to the SAC Ct reference shown.
This study investigated distribution of the SAC Ct, a quality control mechanism in the Xpert® CT/NG assay that reports the number of replication cycles required to reach a detection threshold for the control HMBS gene. We found that lower SAC Ct values were associated with CT and NG infections in urine samples collected from HIV-1-infected women enrolled in NICHD HPTN 040. Each cycle in the PCR process represents doubling the amount of target DNA, this is a two fold increase each time the Ct increases by a value of 1. As each cycle occurs, the amount of DNA will increase exponentially. Therefore a difference in the Ct that may seem small is in fact a very substantial difference in the absolute amount of DNA target. This association of lower SAC Ct values with CT and NG infections was of statistical significance in this patient population recruited from Brazil, South Africa, Argentina and the United States.
Our findings that SAC Ct values in CT and/or NG infected women were lower than those observed in urines of CT and NG uninfected women is suggestive of increased amounts of human cellular material in these urine samples.
Most CT and NG infections are asymptomatic12 and little is known about the risk of consequences and transmission of asymptomatic infections. The SAC Ct may reflect cell turnover, burden of disease and inflammation and therefore should be evaluated clinically with particular focus on the risk for adverse clinical sequelae in asymptomatic patients. The degree to which the SAC Ct value correlates with the burden or severity of infection is unknown at this time and may represent a potential area for future research. If the SAC Ct value is associated with bacterial burden or inflammatory response to infection, this may have implications for predicting the likelihood for adverse sequelae, perinatal transmission, and adverse birth outcomes associated with genital infections like CT and NG. Longitudinal studies are needed to look at associations between SAC Ct values, measures of infection, and clinical outcomes.
This study was subject to some limitations. First, the study population was comprised exclusively of HIV-1-infected pregnant women who were potentially immunocompromised and could have altered inflammatory responses to CT and NG infection, however, most women enrolled in the parent 040 study had asymptomatic HIV infection and CD4 cell counts over 400 cells/mm3. Another limitation is that women in labor or recently delivered may have increased human cellular material in urine because labor is associated with a substantial inflammatory response. Nevertheless, in spite of a recent labor and a potentially immune compromised population, we still found SAC Ct values to be significantly associated with NG and CT infection status. Our study was cross sectional and thus had no follow up information for the women or infants, but sets the stage for future such studies.
The association between SAC Ct values and detection of lower genital tract infections is promising for use with urine samples, but the performance of the SAC will also need to be evaluated using other commonly collected specimen types like vaginal swabs. While further studies are needed, SAC Ct values may show early promise as a marker of clinical diagnosis of genital tract infections such as CT and NG.
The NICHD HPTN 040 study was supported by NICHD Contract # HHSN267200800001C, N01-HD-8-0001 and U01 AI047986 (Brazilian AIDS Prevention Trials International Network), NIAID/ NIH. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. In addition, the study was supported in part by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI), and GlaxoSmithKline on behalf of ViiV Healthcare. The authors would like to acknowledge the assistance of Ms. Mary Ann Hausner and Ms. Jessica Liu who worked diligently in the laboratory in the specimen preparation for the urinary analyses. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Cepheid donated all testing supplies and conducted testing for urine specimen analysis for the detection of infections of Chlamydia trachomatis and Neisseria gonorrhoeae.
Authors declare that there is no conflict of interest.

©2014 Bristow, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.