Journal of
eISSN: 2373-437X


Research Article Volume 2 Issue 2
1Department of Botany and Microbiology, Minia University, Egypt
2Department of Applied and Industrial Mycology, CBS Fungal Biodiversity Centre, Netherlands
Correspondence: Zainab Elsababty, Department of Botany and Microbiology, Faculty of Science, Minia University, P.O. Box 61519, Minia, Egypt
Received: May 09, 2014 | Published: April 25, 2015
Citation: Elsababty Z, Ali AM, Houbraken J. Cellulolytic and pectinolytic enzymes of some selected heat resistant fungi. J Microbiol Exp. 2015;2(2):66-69. DOI: 10.15406/jmen.2015.02.00042
Ten heat resistant fungi, Arthrinium sp., Aspergillus cejpii, A. nidulans, A. spinosus, Byssochlamys nivea, Hamigera avellanea, Talaromyces trachyspermus, T. barcinensis, T. ucrainicus, and Trichoderma asperellum were tested on their ability to produce cellulolytic and pectinolytic enzymes. Except for B. nivea and A. cejpii, all species secreted cellulolytic enzymes in the used cup plate assay. All isolates produced considerable levels of pectinolytic enzymes, when investigated on a liquid pectin medium. The appearance of unexpected blue color in the pectin medium for some species was discussed, but further investigation is required to elucidate this phenomenon.
Keywords: pectinolytic, cellulolytic, heat resistant, fungi, Egypt
Spoilage of heat processed fruits and fruit products due to heat resistant fungi have been frequently reported.1‒3 These fungi are able to survive the high temperatures applied during heat treatment, causing severe economical losses.4 Olliver & Rendle,5 who reported the presence of Byssochlamys fulva in pasteurized strawberries, also studied the action of this fungus on pectin containing substances. Members of the genus Byssochlamys are able to cause fruit disintegration by their ability to produce various pectinolytic and disintegrative enzymes. Ugwuanyi & Obeta6 studied the pectinolytic and cellulolytic enzymes of some selected Neosartorya species (syn. Aspergillus) and other heat resistant fungi, which were isolated from Nigerian soils and tested their macerating effects on two different types of mango. Beaven & Brown7 and Tournas2 have reported that pectinolytic enzymes of heat resistant fungi have been responsible for pectin hydrolysis and consequently texture softening in fruits. Pectinolytic activity is widely distributed among fungi particularly within the genus Aspergillus where it is widely associated with fungal invasiveness.8,9 It is known that in many fungi, the production of cellulases is adaptive in many fungi, which means that the enzyme is not formed in detectable quantities in the absence of cellulose.10 Several workers have reported cellulase activities in rot causing organisms and have sought to associate tissue softening ability with cellulolytic activities.11‒13 The enzymatic digestion cellulosic substrate by fungal cellulases has been proved as economic feasible for the conversion of cellulose into fermentable sugars and fuel ethanol.14,15 Even though there are many reports on cellulase producing fungi,16 only few have sufficiently high activity for commercial success.17,18 Little has been published on the pectinolytic and cellulolytic enzymes of heat resistant fungi. Consequently, current work was undertaken to spotlight on pectinolytic and cellulolytic enzymes of selected heat resistant fungi species.
Isolates
Ten different isolates of Arthrinium sp., Aspergillus cejpii, A. nidulans, A. spinosus, Byssochlamys nivea, Hamigera avellanea, Talaromyces trachyspermus, T. barcinensis, T. ucrainicus and Trichoderma asperellum were previously isolated and identified from soil samples of cultivated fruits, crops, and vegetables in Minia governorate, Egypt. These species were isolated after a heat treatment of 60, 70, 80 and 90°C for time intervals ranged from 10 to 30 min in 20% sucrose Czapek's agar with Rose Bengal (150 mg/l). All isolates were maintained on slants of freshly prepared potato dextrose agar (PDA) at 4°C.
Morphological and molecular identification
All fungal species were identified based on their phenotype and by sequencing ITS regions (incl. the 5.8S rDNA), and then were sent to be identified in CBS-Fungal Biodiversity Centre, Utrecht, The Netherlands. The molecular based identification was performed by sequencing the internal transcribed regions (ITS1 and ITS2) and the 5.8S rDNA. The DNA extraction, PCR and sequencing were performed as described by Houbraken et al.19 The obtained sequences were compared on similarity on Genbank and in internal databases of the CBS-KNAW Fungal Biodiversity Centre.
Production of cellulolytic enzymes in cellulose medium
The tested isolates were cultivated on liquid modified Czapek's medium. This medium contained per liter Carboxyl Methyl Cellulose (CMC), 5g; Na2No3, 2 g; KH2PO4, 1g; MgSO4*7H2O, 0.5g; KCl, 0.5g. The medium dispensed in 10ml portions into 250ml Erlenmeyer flasks was sterilized at 121°C for 15min, then after cooling inoculated by the freshly inoculums of the ten isolates cultivated on PDA, and incubated in an orbital shaker at 30°C and 150rpm for 14 days. Crude enzyme was prepared by the method as described by Ugwuanyi & Obeta,13 the broth culture from each flask was separated from mycelial growth by filtration. The filtrate was dialysed twice against 50 volumes of distilled water at 2-4°C for a day to remove salts and small molecules. Crude enzyme was obtained by centrifuging this filtrate at 5000 x g and 4°C for 15 min. The ten cultures were grown at 28±2°C for seven days on PDA prior inoculation.
The cellulolytic activity of the ten isolates was assayed by the cup-plate method as described by Godoy et al.,20 and Ugwuanyi & Obeta.13 The medium for cellulolytic activity assay contained 5g CMC and 20g Agar-Agar per liter distilled water. Fifteen milliliter quantities of this medium were dispensed into sterile 9cm Petri dishes. Plates were allowed to solidify and a well with a diameter of 5 mm was cut out with a sterile cork borer. This well was filled with 0.2ml of crude enzyme preparation. Assay plates were incubated overnight at 30°C and after incubation flooded with Congo red solution (1 mg/ml) for 15 min. De-staining of the assay plate was carried out with 1M sodium chloride (1M NaCl) for 10-15 min. The degraded CMC was visible as clear zone around the well. The results were recorded as diameter (mm) of clear zone per plate. The assay was repeated three times for every isolate.
Production of pectinolytic enzymes
The isolates were cultivated on pectin medium that was modified from Ugwuanyi & Obeta.6 The pectin medium employed contained: citrus pectin, 5g; Na2No3, 2g; KH2PO4, 1g; MgSO4.7H2O, 0.5g; KCl, 0.5g; per liter. Five milliliters of bromothymol blue was added per liter as indicator (0.5 g/100 ml ethanol). The final pH of the medium before sterilization was 10.5. The modification in this method lies in the removal of agar from the pectin medium and the use of bromothymol blue as an indicator. This was done in order to avoid presence of any traces of carbon sources in the agar, making the citrus pectin the only carbon source in the pectin medium. The pectin medium was distributed in screw-capped tubes (5 ml/tube), and then autoclaved at 121°C for 15 min. The final pH of the medium after autoclaving was 7 (olive color). The test tubes were inoculated in three replicates by inoculum of fresh mycelium cultures of the ten isolates at the surface of the tubes, subsequently incubated in an orbital shaker at 30°C and 150 rpm for 14 days and checked every day macroscopically for the appearance of a yellow color behind the growing mycelium. The color change of the indicator (bromothymol blue) was used to determine the degree of pectinase production. A classification from poor, moderate and strong production was applied. One tube was not inoculated and served as control.
Cellulolytic enzymes of heat resistant fungi
The results of cup plate assay (CPA) of cellulolytic enzymes produced by the ten tested heat resistant isolates are shown in Table 1. It showed the clear zone diameter of cellulase activity varied among the ten isolates ranging from 16 to 26 mm for Hamigera avellanea and Aspergillus nidulans respectively. The results showed that Aspergillus nidulans produced the highest CMCase activity in CMC medium (diameter 26 mm) followed by Arthrinium sp. and Trichoderma asperellum which produced an equal sized zone (23 mm) while the lowest activity was produced by Hamigera avellanea (16 mm clear zone diameter). In comparison with the other species, Aspergillus spinosus, Talaromyces barcinensis, T. ucrainicus and Talaromyces trachyspermus produced moderate activity (22, 21 and 20 mm, respectively). Both Aspergillus cejpii and Byssochlamys nivea showed no cellulolytic activity during this investigation which mean that they did not utilize CMC as the sole carbon source.
|
Isolates |
Clear Zones Diameter (mm)a |
|
Aspergillus nidulans |
26a |
|
Arthrinium sp. |
23 |
|
Trichoderma asperellum |
23 |
|
Aspergillus spinosus |
22 |
|
Talaromyces barcinensis |
21 |
|
Talaromyces ucrainicus |
20 |
|
Talaromyces trachyspermus |
20 |
|
Hamigera avellanea |
16 |
|
Byssochlamys nivea |
ND |
|
Aspergillus cejpii |
ND |
Table 1 Cup plate assay of cellulase activity for ten heat resistant fungi on CMC-agar media
aEach value indicate the mean of three replicates.
ND: Not Detected.
Pectinolytic enzymes of heat resistant fungi
The results of the production of pectinolytic enzymes for the ten tested heat resistant species in liquid pectin medium are shown in Figure 1. The isolates showed varying levels of pectinolytic enzymes in pectin medium. All tested heat resistant fungi produced pectinase enzymes, but a variation in color after the incubation period of 7 days was observed. Hamigera avellanea, Byssochlamys nivea and Aspergillus cejpii had a high pectinolytic enzyme activity in comparison with Aspergillus spinosus and Aspergillus nidulans, which had a moderate activity. The three species of the genus Talaromyces (T. barcinensis, T. ucrainicus and T. trachyspermus) have a low pectinase activity exhibited after 4 to 7 days of incubation. The enzyme activity was measured by the change of the color of the pH indicator bromothymol blue after three days of incubation at 28°C. The control tubes have an olive color (pH 6), and if acid compounds are produced, then the pH indicator turns yellow (pH <6). The production of pectic enzymes varies with pH, and other factors.2
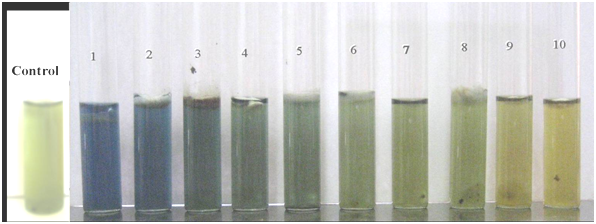
Figure 1 Pectinolytic activity among ten tested heat resistant fungi ordered from left to right.
(1) Hamigera avellanea (2) Aspergillus spinosus (3) Aspergillus nidulans (4) Talaromyces ucrainicus (5) Trichoderma asperellum (6) Talaromyces trachyspermus (7) Aspergillus cejpii (8) Arthrinium sp. (9) Talaromyces barcinensis and (10) Byssochlamys nivea.
By incubation time passing until 7 days, heat resistant fungi in our experiments showed change in color from yellow to blue color, suggesting an increase of the acidity of the substrate. Hamigera avellanea showed a rapid and highest change in color after 4 days of incubation in comparing to Byssochlamys nivea which also gives a high enzymes production but not change in color from yellow to blue. Our results also showed that color of the medium changed from yellow to blue by other isolates but not rapidly.
Eight of the ten tested heat resistant fungi have the ability to produce cellulolytic activity when grown on CMC medium. It is important to note that the cellulolytic assay demonstrated significantly high activity in Aspergillus nidulans, followed by Arthrinium sp. and there was no activity noted with Byssochlamys nivea and Aspergillus cejpii. Our results were found to be in agreement with several other studies, which have reported cellulase activities and its possible role in fruit tissue maceration.21 The absence of cellulase production by B. nivea and D. cejpii are in agreement with another investigation22 who concluded that while cellulase may aid or enhance tissue maceration in some instance, it is not absolutely necessary for the organism to be able to cause fruit tissue maceration. This data is in disagreement with the results of Obeta & Ugwuanyi,6 who found that Byssochlamys nivea was able to secret cellulase enzymes in highest activity whereas N. quadricincta and N. fischeri var. spinosa produced lowest activity.
All of the ten isolates of heat resistant fungi showed the ability to produce pectinolytic activity when grown on liquid pectin medium. Hamigera avellanea exhibited the most rapidly pectinolytic activity among all tested fungi. Furthermore, the results demonstrated that Byssochlamys nivea came second in the pectinolytic activity production. These results are consistent with the Yates & Mooney,23 Chu & Chang,24 Rice & Beuchat25 and Taniwaki,26 they reported that members of the genus Byssochlamys are able to cause fruit disintegration by their ability to produce various pectinolytic and disintegrative enzymes. Pectinolytic enzymes catalyzing the degradation of pectic substances are of great industrial importance.27 These enzymes are required for extraction and clarification of fruit juices and wines, extraction of oils, flavors and pigments from plant materials, preparation of cellulose fibers for linen, jute and hemp manufacture,28 coffee and tea fermentations29 and novel applications in the production of oligogalacturonides as functional food components.30 The results also showed that Trichoderma asperellum, Arthrinium sp., Aspergillus cejpii have the ability to produce various pectinase activities and caused disintegration of the pectin medium. This study demonstrated that Neosartorya spinosa and three species of Talaromyces gave pectinolytic activity, and these enzymes may aid in the spoilage of fruits. These results were in agreement with most investigations which implicated Neosartorya spp., Talaromyces spp., in the spoilage of processed fruits.31‒34 Interestingly, production of pectinolytic enzymes by the tested heat resistant fungi in this experiment showed that Hamigera avellanea, Aspergillus spinosus and Aspergillus nidulans produce alkaline compounds. When pectin is degraded, galacturonic acid is produced and this compound lowers the pH. That the medium becomes alkaline might be due to the fact that galacturonic acid is used as a carbon source for the growth of the fungus. Another option is that the heat resistant fungi produce alkaline metabolites in the liquid pectin medium. These alkaline metabolites may be the toxic secondary metabolites which are produced by some heat resistant fungi such as byssochlamys A, byssochlamyic acid, carcinogenic patulin, the tremorgenic substances, fumiremorgin A and C, fischerin. More work is needed to study this unexpected change from yellow to blue color. Literature highlighting the optimization, biochemical characterization, genetics and strain improvement studies of pectinases from mesophilic fungi35‒39 is available. However, the studies on pectinases from heat resistant fungi are lacking. In developing countries, there is a lack of literatures about pectinolytic enzymes; Al-Gashgari40 has studied of the common fungi occurrence in fruit juices in Saudi Arabia and their ability to produce pectolytic enzymes. Aspergillus flavus, A. fumigatus and A. niger were the highest pectinase production in this study. However, more research about pectinolytic and cellulolytic enzymes of the most occurrence heat resistant fungi in processed juices is in need to be investigated at future.
None.
Authors declare that there is no conflict of interest.

©2015 Elsababty, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.