Journal of
eISSN: 2373-437X


Research Article Volume 12 Issue 3
1Directorate of Document Management and Archives, Ministry of Science, Technology and Environment, Cuba
2Preventive Conservation laboratory, National Archive of the Republic of Cuba, Cuba
Correspondence: Sofia Borrego, National Archive of the Republic of Cuba. Compostela No. 906 esq a San Isidro, Habana Vieja, PO Box: 10100, Havana, Cuba, Tel +53 78629436, Fax +53 7866 8080
Received: June 28, 2024 | Published: July 9, 2024
Citation: Vivar I, Borrego S. Biological diversity detected in two deteriorated Cuban cinematographic films that contribute to their biodegrading. J Microbiol Exp. 2024;12(3):88-96. DOI: 10.15406/jmen.2024.12.00421
The cinematographic films are a reproduced version of reality and have become vital documents to study everything around us. For their preservation it is necessary to study the biodeterioration of these documents. The aims of this study were to characterize the biodeterioration caused mainly by microorganisms in two cinematographic films by applying molecular methodologies, electron microscopy and epifluorescence microscopy as well as to determine the enzymatic characterization of the fungal species isolated. From the Cuban Institute for Cinematographic Industry and Arts (ICAIC) the samples on two damaged cinematographic films were collected. The films were analyzed by different microscopic techniques, including the two types of electron microscopy. Also, the degradative potential of the isolated fungi was determined by qualitative evaluation of the enzymatic activities. A significant fungal colonization on both sides of the films and the damages caused by these microorganisms in the material were observed as well as the exoskeletons of dust mites of the families Tydeidae and Tarsonemidae as part of biofouling that were used as nutrients by the fungi. By epifluorescence microscopy was observed that some of the microorganisms were still viable and active. Using molecular biology techniques could be identified several species corresponding to the fungal genera Aspergillus, Cladosporium, Penicillium and Microascus as well as the bacteria genera Bacillus, Staphylococcus and Kocuria, which were responsible of the biodeterioration of these films. All isolated fungal species were capable to degrade the cellulose and gelatin as well as to excrete organic acids and pigments. Bacteria were detected in low concentrations as well as other biological agents, but it was observed that the films were being colonized by a high concentration of various fungal species with a significant biodeteriogenic potential, demonstrating that they were severely affecting the films.
Keywords: biodeterioration, cinematographic films, electron microscopy, microorganisms, molecular biology
ICAIC (by its acronym in Spanish), Cuban Institute of Cinematographic Art and Industry; ESEM, environmental scanning electron microscopy; SEM, scanning electron microscopy; RD, relative density; aw, water activity
The cultural heritage is the testimony of human creation or of the nature evolution. It is the heritage, the conduit for linking people with their history; hence is important the preserving the cultural heritage for present and future generations.1
The documentary heritage is not without deteriorating and the supports nature determines the degradation type.2,3 Throughout history, people have used various supports, and some examples are stone, metal, papyrus, parchment, paper, glass and various plastics.4,5
The cinematographic films, as part of the documentary heritage, are composed of three elements: a plastic support (cellulose nitrate, cellulose triacetate or polyethylene terephthalate), a substance with silver salts where images are formed and a binder that is the gelatin.6 The last two components are the main materials of the photosensitive emulsion layer. Over time the films suffer deterioration reactions that occur during storage. Among the factors causing the deterioration of the films can be mentioned handling and mechanical damages, but the most important are the physical, chemical and biological damages, which define the direction and speed of the deterioration processes.
The materials making up the films are sensitive to both temperatures and extreme relative humidities and their fluctuations.7 Temperature plays an important role in the degradation of polymers, especially cellulose, therefore the higher the temperature, the faster will be the processes of chemical degradation of the different components of the films. The high relative humidity affects all elements of the film, for example, causes the gelatin layer softens and becomes sticky, thus the film is more vulnerable to mechanical damage, and all this affects the image quality. In parallel, moisture too low can cause deformation and breakage of the film and a detachment of the emulsion layer; this can create static marks during printing or cause buckling due to uneven moisture loss.8
It is also important to consider the damage caused by environmental pollution. Airborne particles such as ash and soot are chemically active products which can degrade the substances over which accumulate especially chemical compounds with silver salts present in the emulsions. Several researchers have studied the processes of deterioration of these materials,7,9,10 and they have focused in the chemical stability of the support. However, in cinematographic films, both, the support as the gelatin are susceptible to microbial attack that can destroy entire film.6,11–13 The bacteria and fungi growth usually can be observed in the emulsion side and the film support as irregular opaque spots.12 So far, there are few studies on microbial contamination in films, highlighting the researches performed in Spain, Italy, Egypt, the Czech Republic and Cuba.10,14–21 This study presents the microbial characterization that affected two films on cellulose triacetate and other biological agents that contributed to their biodeterioration, as well as the biodeteriogenic potential determination of the isolated fungal species through the evaluation of their enzymatic activities.
Sampling
As samples two film color cellulose triacetate films were selected belonging to the Cuban Institute of Cinematographic Art and Industry (ICAIC). The visible effects of microorganisms at the beginning of the rolls and along the edges of the film were considered. Fragments of each film were about 24 cm2.
The repository where the films were stored at the time of sampling did not have the correct condition of preservation to these types of special materials;22 the values of average temperature and relative humidity were 31°C y 65 %, respectively.
Microscopic observations
For an initial impression of the state of biodeterioration of each sample, the fragments were observed by stereomicroscope (Olympus).16,17
Without previous preparation, the film samples were observed by environmental scanning electron microscopy (ESEM) using an INSPECT, QUANTA 200 scanning electron microscope operated at an accelerating voltage of 20e25 kV, to analyze the degree of deterioration of the support and the types of colonizing organisms. Film samples were also observed with scanning electron microscopy (SEM). In that case, the specimens were washed twice with sterile water, fixed with 2.5 % glutaraldehyde in sodium cacodylate 0.01 M at 4°C for 2.5 h, dehydrated with a series of alcohol-water rinses (20, 40, 60, and 80 %), and then submerged in these solutions for 30 min at 4°C. The fixed samples were maintained in a 100 % alcohol solution at 4°C. They were further processed in a critical-point procedure (CPD 030, BAL-TEC) followed by gold sputtering (SCD 005, BAL-TEC) and then observed under a scanning electron microscope (DSM 960, Zeiss) operated at an accelerating voltage of 15 kV.
The viability behavior of the microorganisms colonizing the films was determined by observing the samples by epifluorescence microscopy (Axioskop 2, Zeiss, Germany). They were washed twice with sterile water and were stained using the kit LIVE/DEAD® LIVE/DEAD® BacLightTM Bacterial Viability, that kit distinguishes live cells from dead cell. The live cells are observed in red color or orange while the dead cells in yellow and green.16,17
Isolation and identification of the colonizing microorganisms
To isolate the viable microorganisms responsible for the degradation of cinematographic film A, a small fragment of this film was cut with sterile scissors and added to a tube with 10 mL of distilled water and vortexed for 30 seconds. Of this washing water, 100 µL were seeded in Petri dishes containing three different culture media: potato dextrose agar (PDA) (OXOID), BactoTM malt extract agar (Becton, Dickinson and Co.), and oatmeal agar (Difco) in Petri dishes. Then, that same fragment was scraped from both sides using a sterile metal spatula and 2 mL of distilled water was used to recover the contents. Serial dilutions were made up to 10-4 in sterile saline solution and 100 µL of each dilution were seeded in Petri dishes containing the three culture media mentioned above.
In the case of film B, only the cut fragment was scraped and the procedure described above was followed. This fragment was then placed directly into a Petri dish with PDA.
All samples were incubated at 30°C for 2 weeks. Finally, the colonies were counted to determine their concentration in CFU/mL.
Fungi colonies were purified separately in the same medium from which they were extracted and purified bacteria spreading colonies separately in Tryptone soya agar.
From the isolated fungi the genomic DNA was extracted using the commercial DNEasy Plant mini kit (QIAGEN) following the manufacturer’s instructions. The ribosomal region spanning the internal transcribed spacers (ITS1 and ITS2) and the 5.8S rRNA gene were PCR-amplified using the primers ITS1 and ITS4.23 PCR was performed in a Veriti® Thermal Cycler (Applied Biosystems) with the following program: 5 min denaturation at 95°C, followed by 35 cycles of a 1 min denaturation at 95°C, 1 min annealing at 56°C, a 90 s extension at 72°C, and a final extension step of 10 min at 72°C. All reactions were carried out in a 25 μL volume containing 12.5 μL of ReadyMix_ Taq PCR Mix with MgCl2 (Sigma), 5 μL of DNA (ca. 10-30 μg/mL), 25 pmol of each primer, and 0.4 mM MgCl2. The PCR products were analyzed by electrophoresis in 1 % (wt/vol) agarose gels in 1 x TBE buffer containing ethidium bromide (0.4 μg/mL) and then purified using the enzymatic treatment ExoSAP-IT® (USB) following the manufacturer’s protocol.
From bacteria the genomic DNA extraction was performed by a cyclic process of freezing at -70°C and thawing at 60°C. The 16S rDNA genes were PCR-amplified using the primers 005-F and 531-R.24. PCR was performed in a Veriti® Thermal Cycler (Applied Biosystems) with the following program: 10 min denaturation at 94°C, followed by 30 cycles of a 1 min denaturation at 94°C, 1 min annealing at 55°C, a 2 min extension at 72°C, and a final extension step of 10 min at 72°C. All reactions were carried out in a 25 μL volume containing 12.5 μL of ReadyMix_ Taq PCR Mix with MgCl2 (Sigma), 5 μL of DNA (ca. 10-30 μg/mL), 25 pmol of each primer, and 0.4 mM MgCl2. The PCR products were analyzed by electrophoresis in 1 % (wt/vol) agarose gels in 1 x TBE buffer containing ethidium bromide (0.4 μg/mL) and then purified using the enzymatic treatment ExoSAP-IT® (USB) following the manufacturer’s protocol.
Microorganisms were identified by sequencing of the respective rDNA fragment using the BigDye® Terminator v1.1 cycle sequencing kit (Applied Biosystems). Sequences were resolved in an ABI PRISM® 310 Genetic Analyzer following the manufacturer’s instructions and then compared directly to all known sequences deposited in the databases of the National Center for Biotechnology Information (NCBI), using the basic local alignment search tool Megablast.
Sequences were aligned using the Clustal-X software v. 1.81.25 Phylogenetic and molecular evolutionary analyses were conducted using MEGA (Molecular Evolutionary Genetics Analysis) v. 3.1.26
To identify the dead dust mites settled on the films, detailed observations of their structures were made and the taxonomic criteria described in keys were followed.27–31
Determination of relative density (RD) of the isolated species
This analysis was performed according to Smith (1980)32 where:
RD = (Colonies number of an species / Total colonies number of all species) x 100
Determination of the deteriorative potential of isolated fungal species
The qualitative cellulolytic activity and pigment production of the different isolates were determined by seeded each one on slants with the saline Czapek Agar culture medium without a carbon source. In one case, a strip of Watman paper filter 4.8 cm long by 1 cm wide was used as a carbon source, in other case crystalline cellulose (1 %), and as a control, glucose (1 %) was used. The slants were incubated at 30°C for 21 days. Pigment production was evaluated on the filter paper strip.33
To determine the acids production, spore suspensions of each isolates was made in sterile distilled water and was seeded in the saline Czapek culture broth without carbon source. Glucose (1 %) was used as a carbon source, the pH was adjusted to 7 and phenol red (0.03 %) was added as a pH indicator. The cultures were incubated at 30°C for 3 days, and subsequently the pH of the culture medium was measured using a pHmeter.33
The qualitative proteolytic activity was determined through the hydrolysis of gelatin in culture tubes. Each isolates was inoculated by puncture into the Czapek Agar culture medium without a carbon source. Gelatin was added at 120 g/L and the pH was adjusted to 7.2. The tubes were incubated at 30°C for 7 days. Afterwards, the tubes were stored at 4°C for 1 h and a gelatin hydrolysis reaction was evidenced by medium liquefaction when the tubes were inverted.33
Microscopic observations
Optical microscopy (stereomicroscope) showed obvious signs of biodeterioration in the two films studied, although to varying degrees (Figure 1). While the film A was completely covered by a dense biofilm, the film B showed only dark spots. In addition, it was evidenced that the development of microorganisms, preferably fungi, occurred primarily along the edge of the cinematographic film and perforations, instead of being in the center of the film (Figure 2).
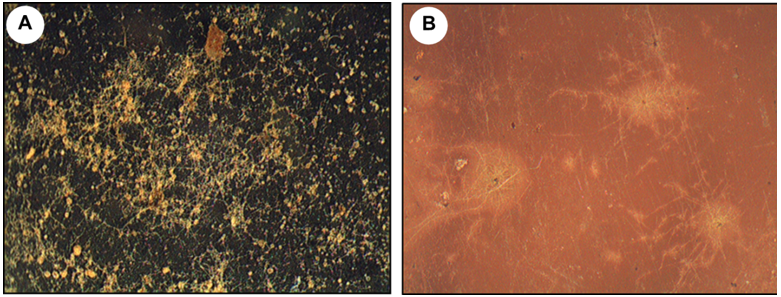
Figure 1 Images obtained from a stereomicroscope (20X) showing microbial colonization of the films analyzed. a) Film A and b) Film B.
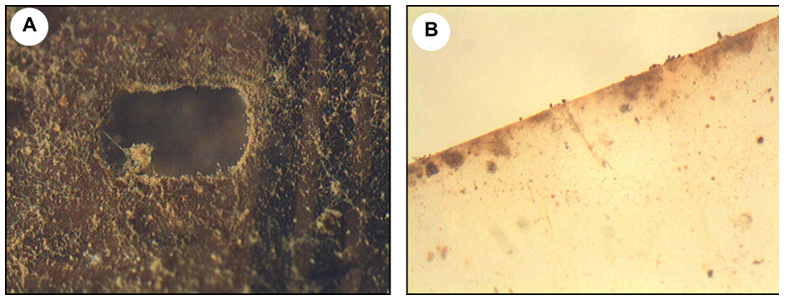
Figure 2 Images obtained from a stereomicroscope (20X) showing microbial growth on: a) the perforations in the film A and b) at the edge of the film B.
Fluorescence microscopy showed that the majority of the growing fungi present on the films were still viable and active (Figure 3). Note some of them in yellow and red, indicative of their viability.
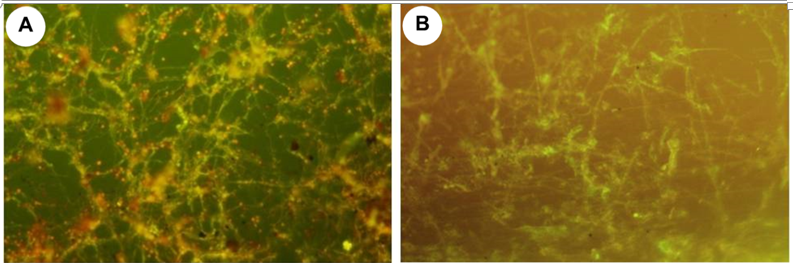
Figure 3 Epifluorescence micrograph of films stained. Viable fungal mycelia can be observed. a) Film A and b) Film B.
The observation made by ESEM confirmed that both sides of the films had microbial growth with differing degrees of colonization (Figure 4) but the microbial growth was different between the films analyzed being the film A was the most contaminated (Figure 5).
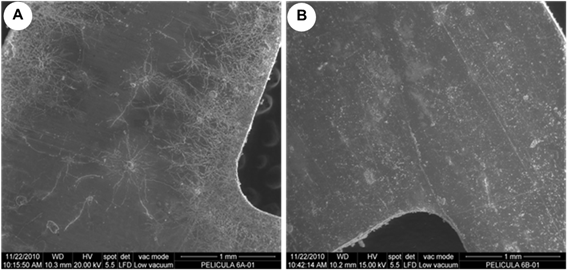
Figure 4 ESEM micrographs showing a different microbial colonization on both sides of the film B. a) side of the gelatin and b) side of the cellulose triacetate.
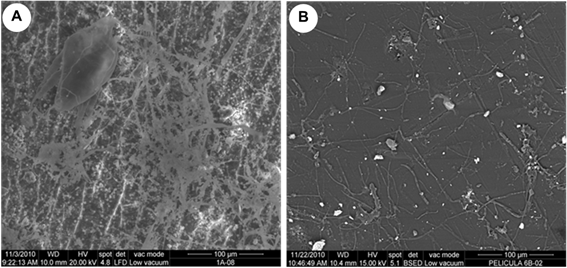
Figure 5 ESEM micrographs showing different microbial colonization in the films on the side the cellulose triacetate. a) Film A and b) Film B.
With SEM also it shows that in the film B fungal hyphae penetrated into the support, causing mechanical damage in the form of cracks independently of chemical deterioration (Figure 6a) and could be detected the extracellular polymeric substances excreted by the fungi as part of the biofilm (Figure 6b). Also, the presence of small holes in the fungal structures as a result of the lytic activity of the bacteria (Figure 6c) was observed.
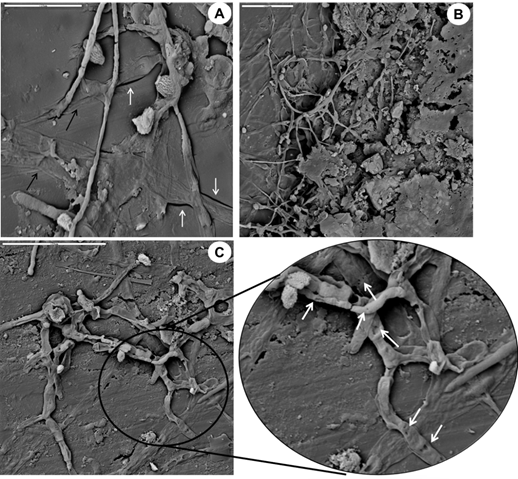
Figure 6 SEM micrographs of the film B. a) Hyphae penetration in the support (black arrows) causing mechanical damage in the form of cracks (white arrows). b) Extracellular polymeric substances produced by fungi as part of the biofilm. c) Small holes in fungal structures (white arrows) resulting from the lytic activity of the bacteria.
In addition to fungi and bacteria, abundant exoskeletons of dust mites were also observed in the ESEM micrographs, as part of biofouling (Figure 7), which contribute to the development of the microbial ecosystem in the films studied and its biodeterioration. Those mite exoskeletons belonged to two families. One of the specimens was an adult of the Tydeidae family, while the other was a larva of the Tarsonemidae family (Figure 8).
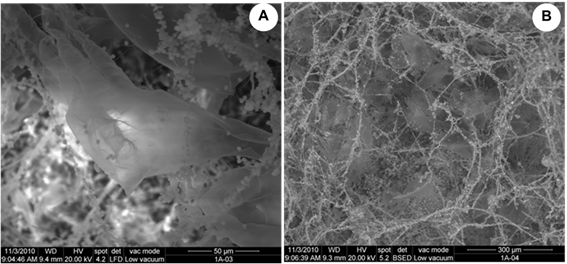
Figure 7 ESEM micrographs showing the biofouling in the film A. The presence of abundant exoskeletons of mites can be observed.
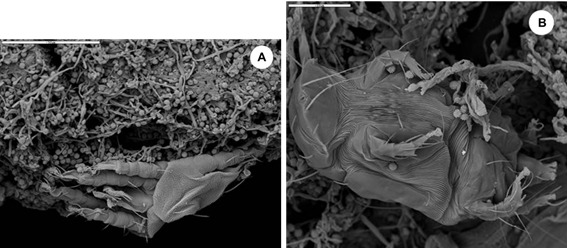
Figure 8 SEM micrographs showing the mite exoskeletons as part of the biofouling existing in the analyzed biofilms. a) Exoskeleton of an adult of the Tydeidae family. b) Exoskeleton of a larva of the Tarsonemidae family.
Identification of viable microorganisms
From washings, scrapes and films fragments, a total of 32 fungi colonies and 10 bacteria colonies were isolated. As from the sequencing of the isolated microorganisms seven species of fungi and five species of bacteria were identified (Table 1).
|
Cinematographic films |
CFU/mL obtained according the origin of sampling |
Identification in the NCBI data base |
|||
|
Washing water |
Scraping water |
Film fragment* |
Fungi species (CFU/mL) |
Bacteria species (CFU/mL) |
|
|
Film A
|
Fungi: 12 x 104 Bacteria: 3 x 10 |
Fungi: 9 x 104 Bacteria: 6 x 10 |
- |
Aspergillus flavus (1 x 104) Aspergillus sydowii (4 x 104) Aspergillus versicolor (16 x 104)
|
Bacillus cereus (1 x 10) Bacillus thuringiensis (1 x 10) Bacillus megaterium (2 x 10) Staphylococcus hominis subsp. hominis (2 x 10) Staphylococcus warneri (3 x 10) |
|
Film B
|
- |
Fungi: 8 x 10 |
Fungi: 2 Bacteria: 1
|
Aspergillus unguis (3 x 10) Cladosporium cladosporioides (1)* (1 x 10) Cladosporium tenuissimum (4 x 10) Penicillium chrysogenum (1)* |
Kocuria koreensis (1)* |
Table 1 Microbial species isolated from the analyzed cinematographic films
*: It indicates that in this case only isolated colonies of the film fragment are reported.
From the film A 21 fungal colonies were detected, 12 in the washing water and nine in the scraping water those belong to three species of the Aspergillus genus. Nine bacterial colonies were detected; three in the wash water and six in the scraping water, corresponding to five species of the genera Bacillus and Staphylococcus respectively. In the film B 12 colonies were detected, 11 of them were fungi belonging to four species of the genera Aspergillus, Cladosporium and Penicillium; eight colonies were detected in the water scraping and three in the film fragment. The other colony isolated of the scraping water, was of a bacterial species of the Kocuria genus.
Among the species of the Aspergillus genus, A. versicolor was dominant species in the film A (Figure 9); although the species A. flavus and A. sydowii were also isolated but in smaller proportion. Aspergillus unguis was another species of the genus, but in this time it was detected in the film B. Other fungal genera found in the film B were Cladosporium and Penicillium. From Cladosporium genus were isolated two species (C. cladosporioides and C. tenuissimum), and only one species from the genus Penicillium was detected (P. chrysogenum).
In the case of bacteria, Bacillus was the predominant genus in the film A with three species (B. cereus, B. thuringiensis and B. megaterium) followed of the Staphylococcus genus with two species, S. hominis and S. warneri. In the film B only one species was isolated and belongs to the Kocuria genus (K. koreensis) (Figure 9).
Enzymatic characteristics of the isolated fungal species
Enzymatic characterization of fungal isolates revealed that most of them grew at the expense of filter paper as the only carbon source (this source is a mixture of a and β cellulose) and that most of the species were capable of degrading the paper cellulose with varying intensity as well as the crystalline cellulose (a-cellulose) (Table 2). Similarly, all species excreted acids and degraded the gelatin, but only a few of them excreted pigments onto the filter paper strip (57.1 %). It is noteworthy that the C. tenuissimum species was the only one that showed low cellulolytic activity and that practically did not produce acids (pH value very close to 7) despite being capable of degrading gelatin and excreting an olive-colored pigment, while C. cladosporioides was a species that revealed good cellulolytic and proteolytic activities, in addition, it was also capable of excreting a large amount of acids and an olive-colored pigment. Of the Aspergillus species, although A. sydowii and A. versicolor showed high cellulolytic activity, A. flavus excreted the greatest amount of acids, since it was able to greatly lower the pH of the culture medium.
|
Species |
Cellulose degradation |
Acid production (pH) |
Pigment production1 |
Proteolytic activity (gelatin) |
|
|
Growth on filter paper |
Growth in crystalline cellulose |
||||
|
Aspergillus flavus |
+ + + |
+ + |
3.88 |
+ (amber) |
+ |
|
Aspergillus sydowii |
+ + + |
+ + + |
5.39 |
- |
+ |
|
Aspergillus versicolor |
+ + + |
+ + + |
5.39 |
- |
+ |
|
Aspergillus unguis |
+ + + |
+ + |
5.83 |
+ (very light amber) |
+ |
|
Cladosporium cladosporioides |
+ + + |
+ + |
3.93 |
+ (olive tone) |
+ |
|
Cladosporium tenuissimum |
+ |
- |
6.91 |
+ (olive tone) |
+ |
|
Penicillium chrysogenum |
+ + + |
+ + |
5.79 |
- |
+ |
Table 2 Enzymatic characteristics of the isolated fungal species
1The production of pigments was observed on the filter paper strip. + + +: Indicates abundant growth. + +: Refers to moderate growth. +: Specifies poor growth, it is also indicative of the pigment excretion and the liquefied gelatin. –: Indicates NO growth and NO pigment production.
Microscopic observations showed that the greatest biological contamination was found mainly at the edges and perforations of the films and the fungi predominated. Similar results were obtained by Abrusci12 in a study performed in films from the cinematographic archives of Madrid, Las Palmas and Barcelona in Spain. These areas were more susceptible to colonization, possibly because they retain the environmental moisture and possess greater oxygen availability; also are the areas of greatest manipulation. In any case, once contaminated the film, if environmental conditions are suitable for growth to continue, image damage could be permanent. On the other hand, through epifluorescence microscopy it was proven that the majority of the fungi colonizing the films were still viable and active, so they can continue the deterioration process of these supports. This result clearly shows that the biodeterioration is a continuous process that can cause permanent damage to the supports under study.
Furthermore, with the SEM it was possible to reveal the presence of bacteria indirectly since the holes that they caused in the fungal structures were observed. A similar result was obtained by Florian and Manning34 to study the foxing in the documents.
The phylum Ascomycota predominated among the isolated fungi.18,35,36 Of them, Aspergillus was the predominant genus followed by the genera Cladosporium and Penicillium. These genera were isolated previously from Cuban films16,37 and Spanish,12 from photographic negatives,38 and photographs preserved in Cuba,39 Italy,35,40 Egypt,41 Russia42 and Poland,36 as well as from glass plate negatives43 and contaminated photographic and cinematographic materials conserved in various archives of the Czech Republic.18,20 Also were reported as commonly predominant genera in indoor environments of archives, libraries and museums of Cuba44–49 and other countries.50–53
The genera Aspergillus and Penicillium are considered primary colonizers, as they can grow at low values of water activity (aw < 0.8) while other fungi genera such as Cladosporium, which it is considered as secondary colonizers, require aw values of at least between 0.8 - 0.9;8s,54,55 so, in tropical climates, such as Cuba, where the relative humidity is very high, it is easy to explain the presence of these genera.
Within the Aspergillus genus, A. versicolor was the predominant species in the film A as in previous reports.11,15,37 This species was also isolated from photographs13,42 and glass plate negatives with gelatine as emulsion.43 Furthermore, A. sydowii and A. flavus were also isolated lesser extent. Aspergillus unguis was another species identified within the genus, but corresponding to the film B. These species were also previously isolated from ICAIC cinematographic films by Gomez.37 The behavior obtained in the film A was expected, since this film was the most biofouling and was the most biological diverse in that biofouling. Through SEM it was evident that this film had many exoskeletons of dust mites, which served as additional nutritional source for the development of an abundant and thick fungal biofilm.
Cladosporium was the highest prevalence fungal genus in the B film. In this case of two species were identified, C. cladosporioides and C. tenuissimum. This genus was detected previously in ICAIC cinematographic films37 and the other countries,20 as well as in audio-visual materials18 and in photographs.33,38,40,56,57 Cladosporium cladosporioides is a species that has been detected in cinematographic films12,58 and photographs.33,42 It is also known that this species have been isolated from the dust by their ability to withstand certain levels of dryness since it is considered a lightly xerophilous species;47,59,60 although generally the representatives of this genus require moisture to grow.54,55 Cladosporium tenuissimum is a species that can be found in the air and collected dust in National Archive of the Republic of Cuba47 and other Cuban archive,45,49 but it had never before been isolated from a document preserved in a Cuban archive or library. Of the Penicillium genus, the only species isolated in the B film was P. chrysogenum. This species was also previously isolated from cinema films,12,15,58 audio-visual materials18 and photographs.33,56,61
All these species have been previously found in the air of Havana city,62–64 which suggests that they were able to penetrate inside the archive where these two films were preserved and be deposited on them; and under conditions appropriate for their development, they grew and formed extensive and strong biofilms on these films.
These fungal species are characterized by degrade gelatin (a compound present in the film binder) and excrete organic acids,33,61 contributing to the deterioration of cinematographic films.8,65,66 But given that the existence of several biodegradation attributes in a single fungal strain is an important factor that determines its ability to cause severe damage to archival collections,33 this analysis was conducted. In this case it was only possible to highlight two combinations. The sum of the species with four (42.9 %) and three (57.1 %) attributes indicated that all showed a high capacity to degrade organic substances and their derivatives that make up the cinematographic films. This demonstrates that the isolated fungal species have significant biodeteriogenic potential and therefore, they can affect the films severely.
For bacterial contamination, Gram-positive bacteria were dominantly isolated. Particularly, the most represented genera were Bacillus and Staphylococcus, followed by Kocuria. These results are consistent with those of other authors who suggest that in the film manufacturing process, bacteria can become trapped in the gelatin and remain viable until the environmental conditions allow them to grow.12,67 De Clerck and De Vos67 reported that in the final extract gelatin almost all contaminating bacteria belonged to the Bacillus genus. Among the species of this genus were found B. megaterium, which was also isolated from Spanish films12 and, according Abrusci et al.,14 it was one of the species responsible for the formation of biofilms on film materials polyester, together with B. cereus. These two species were also found in the film A.
Within Staphylococcus genus were identified the species S. hominis and S. warneri. From these, S. hominis it was reported in previous studies.12 These species are the most important for their persistence in human skin,12,68 so they may have reached the films as a result of poor handling. It is important to note that the genera Bacillus and Staphylococcus have been previously isolated of the photographs conserved in a Cuban archive69–74 and in other countries,21,40 as well as from Greek documents in paper,75 photographic and cinematographic materials20 and audio-visual materials.19 It has been reported that these bacterial genera can penetrate the indoor air of premises along with dust68,76 can also be transported by shoes of the people and the dust it is suspended on the floor and that is disperses when people walk on it.77 Members of the Kocuria genus, were isolated previously from films,12 photographic and cinematographic materials,20 photographs56 and paper documents.75,78
The high frequency of these Gram-positive bacteria genera coincide with the study conducted by Purkrtova et al.,20 where, most frequently Gram-positive cocci such as Staphylococcus and Kocuria genera, and Gram-positive rods forming endospores such as the genus Bacillus were obtained.
Similarly, to the fungal genera Aspergillus, Cladosporium and Penicillium, the most often isolated bacterial genera were also isolated across most archives audio-visual materials (principally photographs and films), such as the genera Bacillus, Staphylococcus and Kocuria. This could suggest the hypothesis that air and dust should also be considered as the main source of contamination of these fungi genera. As Staphylococcus and Kocuria genera are important parts of the normal human skin bacteriome, this may suggest that contamination of these materials may have been due to direct contact with human skin by manual handling, but was not shown to be the dominant contamination vehicle.
Regarding dust mites, it has been seen that representatives of the two families detected in this study are very common in Cuba79,80 as well as in other countries.81,82 On the other hand, members of these two families could be previously detected in the dust collected in the National Library of Cuba José Martí.83 It is known that members of the Tydeidae family are cosmopolitan, soft-bodied, striated or reticulated mites that are reported to be mainly phytophages, mycophages, pollenophages, insect parasites or scavengers and nematode predators.31
On the other hand, it is known that members of Tarsonemidae family comprises a relatively high group of mites having a greater diversity of feeding habits than any other Acari family including fungus feeders, plant feeders, and parasitic/symbiont associates of insects. Phytophagous species feed on leaves, flowers, fruits and soft stems that some of them are important pests of agricultural crops.84
About the biodegradation of materials by fungi can affect the material integrity of the same because the fungus’s hyphae penetrate the substrate. Damage can also occur as a result of enzymatic action. Fungi can produce a wide range of enzymes (proteinases, gelatinase, cellulases, etc.) which are able to destroy the component substances of the films.8,33,39,65,85 Also, fungi can be considered risky for documental preservation if it has at least one biodegradation attribute that compromises the document status;73 however, combining them in a single isolates, can mean greater potential to colonize the materials in different ways accelerating its deterioration. From the ecological point of view the fact that the same fungi present more than one biodegradation attribute means that can be developed on the different components of the films, facilitating its prevalence in the ecosystem over other microorganisms (e.g. bacteria) with fewer possibilities.86 According to the obtained results, it was shown that the isolated fungal species can degrade all the substances that make up the films.
When cultural heritage is attacked by microorganisms, the persistence and extinction of the bacterial and fungal species colonizing the objects can vary with time and depends on the environmental conditions and the availability of nutrients and eventual competition with other species for their use. Moreover, the biological characteristics of the species are not the only reasons for fungal and bacterial successions, but rather biological patterns and processes play a decisive role in the way by which species are assembled in both space and time.86 However, recent evidence indicates that mechanisms are more complex and that often the co-occurrence of bacteria and fungi is the norm.20,38,87
The results obtained in the studied films expected, since at the time of sampling were stored in a repository without air conditioning and the average values of temperature and relative humidity were 31°C and 65 % respectively. In the repository had a strong smell of vinegar due to the presence of volatile organic acids such as acetic acid and propionic acid which are emitted during the chemical deterioration of the films of cellulose acetate called “vinegar syndrome”,8,18,20 indicating a high probability that the supports were acids. The films were not protected in adequate packaging and the way they were placed did not allow for systematic cleanings. Thus, environmental conditions, biota and environmental chemical contamination present on the site in conjunction with organic and existing inorganic matter in the films favored the development of destroying organisms that used organic matter for nutrition and changed the structure the cinematographic films. This is the case, first fungi on environmental conditions listed and nutrient availability in the films were easily developed and formed a dense biofilm as observed by ESEM, even was observed that they remained strongly adhered to the surface of films after treatment of the SEM. It is also the case of the bacteria that are active but latent state (speed delayed growth), since are disadvantages regarding fungi because they require for their development of higher water activity and a neutral or slightly basic pH, aspects that were not favorable for its development; although they surely also metabolized the components of the films and the other nutrients provided by the fungi. In general, the damage caused by bacteria is less than those provoked by fungus under these conditions. If each piece of film were considered as an ecosystem, it can be suggested that the existence of consumer organisms knew how to take advantage of the living material provided by other organisms that directly damaged the support. Such is the case of mites that feed on fungi, and these exoskeletons and excreted substances served, in turn, as additional nutrients for the fungal growth. Likewise, these fungi were a nutritional source for bacteria, thus giving rise to an ecological succession that is summarized in figure 10.
It is important to highlight that all the biological agents detected in these two cinematographic films can affect human health. Mites, even dead, can cause severe allergies in humans. They are powerful allergens82,88 and their remains or debris is also potent allergens.88 On the other hand, fungi are also important allergens for humans89–91 and many species that have very small spores, such as those belonging to the genera Aspergillus and Penicillium, as well as those of the species C. cladosporioides, can reach the bronchi and alveoli when breathed and/or inhaled;92–94 therefore, they can trigger deep mycoses depending on the quality of the individuals' immune system.
In the biological characterization of the two deteriorated cinematographic films microorganisms (bacteria and fungi) of diverse species and with different levels of viability could be found, as well as mite exoskeletons that were being used as nutrients by the fungi and those in turn were used by the bacteria as nutrients, demonstrating the complex ecosystem that can develop in documents where biodeterioration is occurring. Furthermore, it could be demonstrated that the different fungal species detected were metabolically active and degrading the films.
The authors thank Dr. Josefina Cao for her contribution in the identification of the dust mites detected on the film films analyzed.
Authors declare that they have no conflict of interest.

©2024 Vivar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.