Journal of
eISSN: 2373-437X


Research Article Volume 8 Issue 1
1Department of Applied Chemistry, Defence Institute of Advanced Technology, India
2Department of Materials Engineering, Defence Institute of Advanced Technology, India
Correspondence: Kajal S Landage, Department of Applied Chemistry, Defence Institute of Advanced Technology, Girinagar, Pune-411025, India
Received: January 02, 2020 | Published: February 4, 2020
Citation: Landage KS, Arbade GK, Khanna P, et al. Biological approach to synthesize TiO2 nanoparticles using Staphylococcus aureus for antibacterial and antibiofilm applications. J Microbiol Exp. 2020;8(1):36-43. DOI: 10.15406/jmen.2020.08.00283
Nano-sized materials have been an important tool in basic and applied sciences. A novel, low cost, green and reproducible bacteria, Staphylococcus aureus mediated biosynthesis of titanium dioxide nanoparticles (TiO2 NPs) was reported in the present study. Initial conformational studies were done using UV-visible spectroscopy and confirmed the synthesis of TiO2 NPs in the broth. The detailed characterization of the TiO2NPs was carried out using SEM, XRD, FTIR and Raman spectroscopy. From the SEM, it was confirmed that the sample showed the NPs were smooth and spherical with an average diameter of about 20nm. From FTIR analysis, it was confirmed that the TiO2 nanoparticles are crystalline in nature, which was confirmed by the FTIR peak at 518cm-1 corresponds to the TiO2 vibration present in a crystalline structure. Additionally, the synthesized NPs were also characterized by Transmission Electron Microscopy (TEM) and Particle size analyzer. This study was aimed to determine the antibacterial and antibiofilm activity of Titanium oxide nanoparticles against both Gram-positive and Gram-negative bacterial species and significant positive results against Bacillus subtilis and Escherichia coli were observed.
Keywords: Staphylococcus aureus, titanium dioxide nanoparticles, antibacterial, antibiofilm
Nanoparticles are quite often employed in the field of nanotechnology which has become the current research trend. However, chemical compositions, sizes and high monodispersity are one of the challenging issues in nanotechnology for the development of reliable protocols for the synthesis of nanoparticles.1,2 An important area of research in nanoscience deals with the synthesis of nanometer-sized materials of different morphologies, sizes and with mono dispersion.3,4 In modern nanotechnology, the interaction between inorganic nanoparticles and biological structures are one of the most exciting areas of research. But there is a need to develop an eco-friendly approach for nanomaterials synthesis from the point of view of environmental health and social aspects. Different microorganisms produce inorganic materials either intracellularly or extracellularly.5,6 Nanotechnology deals with materials having the size in the range of 100 nm. NPs show different properties and depending on the properties it has been used in different domains for various applications.6‒10
The microorganisms are used as possible “nano factories” for development of clean, nontoxic and environmentally friendly methods for producing nanoparticles. Various chemical and physical methods are employed for the synthesis of nanoparticles.11‒13 The synthesis of nanoparticles may cause adverse environmental and health effects.13,14 There are different methods for the synthesis of TiO2 NPs such as sol-gel, hydrothermal, fungal mediated biosynthesis etc. The production of silver nanoparticles within the periplasmic surface of Pseudomonas stutzeri, the formation of gold nanoparticles using Salmonella typhi.15‒18 and C. limone were demonstrated practically using the above technique.19,20 Biological synthesis are beneficial when compared to synthetic methods as biological methods are more eco-friendly.20‒22 The biological method for nanoparticle synthesis is simple, eco-friendly and allows for getting controlled size nanoparticles which can be used as catalysts with specific composition, which is not possible by classical methods. Applications in sensors and medicine are envisaged and the nanoparticles synthesized by using bacterial strains can be further used for biomedical applications.
Titanium, for its weight property and strength, has been used in various industries for a variety of applications. It is lighter than steel with good mechanical strength and twice as strong as aluminium.19,20,23 In the present study, the above aspects have been considered, in order to develop an ecofriendly biotechnological approach for the production of TiO2NPs. It has also been found in the literature that lactobacillus has been used for synthesis similar synthesis.24 Due to its properties among other oxide semiconductors, TiO2 has been more in use in a variety of industrial applications.25 TiO2 is a great material for photocatalysis and development of various biomaterials.26 The biocompatibility property of titania allows its use in bone tissue engineering for the regeneration and healing of damaged bone.27‒29 TiO2 is an efficient catalyst, can be used further for the removal of numerous environmental pollutants and it has been demonstrated for water and surface cleaning.11,29,30 In the present study, we have synthesized TiO2 NPs by using S. aureus. The antimicrobial and anti-Biofilm property of TiO2 NPs was utilized here for the biomedical application. A typical scheme of the work is represented in Figure 1.
Materials
Titanium tetraisopropoxide (TIP) with an average molecular weight 284.25g/mol and ethanol were purchased from Sigma Aldrich, India. Nutrient agar, Nutrient broth and bacteriological Agar powder were purchased from Himedia, India. All the chemicals, solvents and reagents used in the study were of analytical grade and used without any further purification. The bacterial strains (Escherichia Coli, Bacillus subtilis and Staphylococcus aureus) used during the present studies were obtained from the National Chemical Laboratory, Commercial Unit, India.
Synthesis of TiO2 nanoparticles using S. aureus
S. aureus cells were allowed to grow as a suspension culture in 100ml sterile nutrient broth medium for 36h. This culture was treated as source culture. 25ml of source culture was taken and diluted four times by adding 75ml of sterile nutrient broth medium. This diluted culture solution was again allowed to grow for another 24h. 20 ml 0.0025M [Ti(OH)2] titanium tetraisopropoxide was mixed to the broth culture and kept in a steam bath for ~20min at 60°C, As white deposition was observed bottom of the flask, indicating transformation initiation. The broth culture containing S. aureus was incubated at room temperature. After 12-48 h, the broth culture was observed for distinctly markable coalescent white cluster deposited at the bottom confirming the synthesis of TiO2NPs.
Characterization of TiO2 nanoparticles
The Synthesis of TiO2 nanoparticles in the broth medium was confirmed by taking UV absorbance by Biospectrotometer (Eppendorf, Germany). The X-ray diffraction (XRD) patterns of the samples were measured by using X-ray diffractometer with Cu-Kα radiation. The crystalline nature and average crystallite size of the TiO2 nanoparticles were recorded using X-ray diffraction (XRD) (Bruker, Germany) with CuKα radiation (1.5406 Å) in the 2θ scan range of 10-90°. To investigate the morphology and diameter of the nanoparticles SEM was conducted using FE-SEM (ZEISS, Germany). FTIR spectrum of TiO2 nanoparticles was recorded on Fourier Transform Infrared spectrophotometer (Bruker, Germany) in the region of 4000 to 500 cm-1. The surface morphology of the NPS was observed using SEM and AFM. While the average diameters and size distributions of TiO2 NPs were calculated by counting over 50 particles from the SEM Micrographs by ImageJ software. The excitation and photoluminescence (PL) spectra of the sample were recorded on a spectrophotometer (Agilent Technologies) in the range of 250nm to 800nm with an excitation wavelength of 320nm. The average particle size distribution was carried out using Particle size analyzer. Raman spectra were recorded using EZ Raman spectrometer in the range 4000 to 400cm-1 Whereemitted wavelength is 780nm. The photocatalytic activities were done under UV applying 254nm and 365nm irradiation.
Antibacterial properties of TiO2 nanoparticles
The antibacterial activity of TiO2 NPs was studied by disc diffusion method.31 The desired concentrations of TiO2 were prepared by dissolving the appropriate amount TiO2 NPs powder and dissolved in DI water. The whatman filter paper discs were prepared of 3mm diameter, sterilized and then added to the TiO2 solution. Simultaneously, the nutrient agar plated were prepared seeded with the bacterial cultures and allowed to grow for overnight at 37°C along with the discs loaded with TiO2 NPs. After incubation, the plates were observed for the inhibition zones and recorded.
Effect of TiO2 nanoparticles on bacterial biofilm formation by tube method
The assessment of biofilm formation qualitatively was determined by tube method. Simply, Brain Heart Infusion Broth (BHI) + sucrose (2%) +0.5ml TI-NP (15mg/ml) was used for bacterial inoculation. BHI/sucrose inoculated plate was used as a control. Both the plates were incubated at 37°C for 24 h in bacteriological incubator. To study the biofilm-forming potential the glass tubes were decanted and washed with distilled water and dried tubes were stained with crystal violet (0.1%). Excess stain was removed and tubes were washed with de-ionized water. The biofilm formation was observed by drying tubes in an inverted position. Biofilm formation was considered positive when a visible film lined the wall and bottom of the tube. Ring formation at the liquid interface was not indicative of biofilm formation.
UV–visible spectra analysis
In order to confirm the synthesis of TiO2NPs, the UV absorption was checked. Figure 2 shows the UV–Visible spectra for the TiO2 nanoparticles. The UV spectra show a distinct absorption peak confirming the anatase phase of nano-TiO2. The cut off wavelength of TiO2 nanoparticles was observed at 324nm. It is matching with the previous studies of our research group.32 The band gap of TiO2 was calculated using equation 1.
(Equation 1)
Where, h=Plank constant (6.626x10-34Joules sec), c=Speed of the light (3.0x108meter/sec), k=cut off wavelength (410.57x10-9 meters). The band cap value of the TiO2 was found to be 3.88eV. In the present study, the bandgap value (3.88eV), which is close to those reported for the anatase TiO2 nanoparticles.
Scanning electron microscopy (SEM): The surface morphology, shape and size of NPs were analyzed by SEM. Figure 3 shows SEM images of TiO2 nanoparticles synthesized by using a bacterial strain S. aureus. The nanoparticles were spherical, oval in shape, smooth surface and having an average diameter of around 20nm.
Particle size analyzer: Figure 4 represents the particle size distribution of TiO2 NPs. The particle size of the nanoparticle ranges in size from 10 to 30nm which is a good agreement with the SEM results. The uneven distribution of NPs can be clearly observed.
FTIR analysis: Fourier transform infrared spectroscopic analysis was performed to apprehend the interaction of NPs with capping agents. The FTIR spectra of TiO2 nanoparticles show the presence of broadband at 3000–3500cm-1 corresponds to stretching vibration of terminating hydroxyl groups in samples. The spectra of TiO2NPs exhibited prominent peaks at 2923,2856,1649,1545,1450,1393,1234, 1069 and 679cm−1 (Figure 5(a)). The main peaks due to C–H symmetric and asymmetric stretching, C=O stretching and C–O stretching frequencies were observed near 2923cm-1, 1638cm-1, 1069 cm-1 respectively. The Peaks at 1450, 1393 and 1164 were assigned for bending vibrations of primary and secondary amines. The peak at 2923cm−1 corresponds to carboxylic groups. The peak 2856cm-1 corresponds to C-H stretch,1649cm-1 corresponds to C=O stretch of amines. The peaks at 2923, 1649 and 679cm−1 are observed in the spectra due to the biogenic substances (lipids and proteins) associated with the synthesis of TiO2 nanoparticles. In addition, peat at the 679cm-1 corresponds to metal binding to carboxylic groups proposed that the proteins could bind with nanoparticles through the free amines groups or crystalline residues in the proteins, might help in the nucleation of nanoparticles formation.3
Photoluminescence studies: To understand the luminescence properties of synthesized nanoparticles, the photoluminescence analysis has been employed. It was observed that the emission band for NPs was about 390nm. The light-emitting property of nano-titania may rise due to the existence of surface plasmons. Figure 5(b) shows a stokes shift of 70-76nm with reference to absorbance band (∼3.88eV, 324nm). Therefore, rules out the emission in the visible light range due to the absence of free Ti-OH states.32
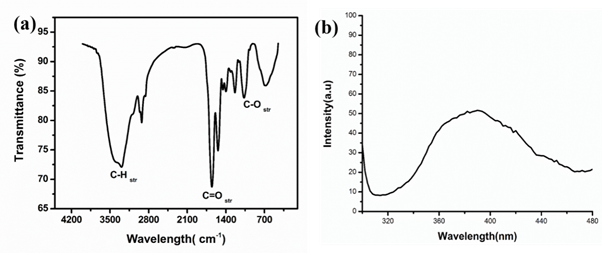
Figure 5 (a) FTIR analysis of TiO2 NPs synthesized by using TiO2 NPs and (b) Photoluminescence spectra of TiO2 NPs synthesized by S. aureus.
Atomic force microscopy: The AFM was performed to have more insight into the topological map of the surface and agglomeration of TiO2 NPs. The AFM obviously depicts the formation of the rutile and anatase forms in the TiO2 NPs, and the surface morphology of the particle which was uneven due to the presence of some aggregate and individual particles. AFM offered a three-dimensional visualization of TiO2. The strong crystalline nature can be observed in the form of diagonal formations with ridges (Figure 6). By means of AFM no linear trend in roughness was observed, but it is proved that the highest TiO2 concentration results in the formation of a smoother layer. In AFM analysis it was observed that the TiO2 NPs were in the size of 10-20nm which was in good agreement with SEM and XRD data.18,29,33
XRD analysis: The crystal phase of the TiO2 was confirmed using XRD. The XRD patterns of the NPs synthesized by using the bacterium Staphylococcus aureus is shown in Figure 7(a) The appearance of sharp diffraction patterns designates the small size, crystalline and high purity of the synthesized sample.34
The line broadening of the diffraction peaks shows that materials were in the nanometer range. The diffraction patterns showed that the anatase phase (a=3.782 A˚, JCPDS no: 84-1286) was formed.35 The prominent peaks obtained in the XRD pattern of the bio-mediated synthesis of TiO2 nanoparticle after removing organic impurities by heating. The peak signals at (101), (103), (004), (112), (200), (105), (211), (204), (220), (215) and (224) planes confirm that the formation of anatase crystal phase mostly, which coincides with JCPD 89-4921 standard.17Average particle size was calculated using Scherer’s equation (equation 2):
(Equation 2)
Where λ is the X-ray wavelength, typically 1.54 Å, K is the shape factor, (0.9), β is the line broadening at half the maximum intensity (FWHM) in radians, θ is the Bragg angle, τ is the particle size. The size of the bio-mediated TiO2 particle was found between 17.01-18.19nm. The 2θ at peak 25.29° confirms the TiO2 anatase structure. Strong diffraction peaks at 25° and 48° indicating TiO2 in the anatase phase. There were many other diffraction peaks found in the sample that might because of bacterial cell proteins. The 2θ peaks at 25.29° and 48.21° confirm its anatase structure.
Raman analysis: The structural properties of the TiO2NPs were further investigated by Raman spectroscopy. Figure 7(b) represent the Raman spectra for TiO2 nanoparticles. The spectrum was typical of the anatase TiO2 phase confirms the phase obtained from XRD. According to factor group analysis, anatase has six Raman active modes (A1g + 2B1g + 3Eg).36 The Raman spectrum of an anatase single crystal has been investigated by Ohsaka and others36 who concluded that the six distinct active modes appeared at 136cm-1 (Eg), 190cm-1 (Eg), 388cm-1 (B1g), 504cm-1 (A1g), 516cm-1 (B1g) and 630cm-1 (Eg). The Raman spectra indicate that the anatase shell was crystallized; a Raman shift at 136cm-1 can be attributed to the dominating Eg vibrational mode in anatase TiO2. The main features of the spectra of the sample are very similar to those of the reference TiO2, which means that the anatase phases of the nanoparticles of samples possess a certain degree of long-range order. Comparing the obtained Raman spectra with the available literature.37,38 It is clear that the Raman bands shift towards higher wave number and their intensities relatively decrease as the particle size decreases.
Transmission electron microscopy (TEM): It is shown that the as-prepared powder was completely crystalline and entirely consists of anatase phase. Due to the relatively poor contrast in the micrograph, it is difficult to exactly measure the size of the primary spherical particles accurately. From the micrograph, their diameter is estimated to be below 20nm, which is in good agreement with the XRD results. The first four rings are assigned to the (101), (004), (200), (005) reflections of the anatase phase.
Figure 8 shows the TEM image of TiO2 nanoparticles. It is elucidated from the figure that the TiO2NPs were agglomerated; mostly spherical in shape and size of particles was in the range of 10–20 nm. This result supports our XRD data in determining the particles size, which coincides with a TEM image. The lattice fringes calculated as 0.327nm which are in good agreement with the previous reports for anatase phase titania NPs respectively.17,32,39
Antimicrobial activity of TiO2 NPs: Figure 9 shows the antibacterial activity of TiO2 nanoparticles, it was carried out by disc diffusion method against two bacterial strains viz. Escherichia coli and Bacillus subtilis. The formation of inhibition zones around the TiO2 nanoparticles integrated discs clearly shows the sensitivity of the bacterial species to TiO2 nanoparticles. The diameter of the inhibition zone was measured for each bacterium, shown in Table 1. The differential sensitivity of Gram-negative and Gram-positive bacteria towards nanoparticles may be depends upon their cell outer layer attribute and their interaction with the charged TiO2 nanoparticles. It was observed that Gram-negative bacteria are more sensitive than Gram-positive bacteria. This property of TiO2 NPs can be utilized in coating the medical devices like catheters to avoid the opportunistic pathogenic infection.
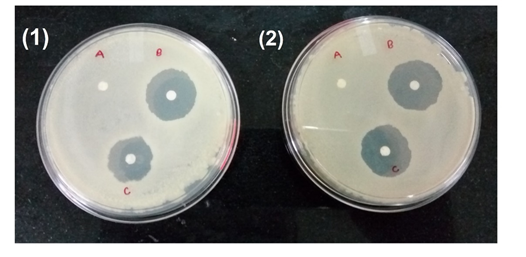
Figure 9 Antibacterial activity of TiO2 NPs (1) against B. subtilis (A) Control (B) 15mg/mL TiO2 NPs (C) 10mg/mL TiO2 NPs s (2). Against E. coli NPs (A) Control (B) 15mg/mL TiO2 NPs (C) 10mg/mL TiO2 NPs.
|
S. No |
Bacterial Strain |
Zone of Inhibition (mm) |
|
TiO2 NPs |
||
|
1 |
E. coli |
14 |
|
2 |
B. subtilis |
9 |
Table 1 Zone of inhibition against bacterial strains by TiO2 NPs
Effect of TiO2 NPs on bacterial biofilm formation
Biofilm forming potential of bacterium increases its pathogenicity and increases the chances of opportunistic infections, which are extremely difficult to eliminate. Biofilm forming strains show resistance to antibiotics. The ability of a bacterium to form a biofilm, they are found as a common contaminant in medical devices like catheters. The immune compressive host is very prone to the infections by such pathogen.TiO2 NPs moving beyond viability and growth; a functional assessment performed examining specific function. The biofilm formation is a common phenomenon in bacteria especially has a role in quorum sensing etc. A comparative study was done to study the biofilm formation in Bacillus subtilis and E. coli with and without nanoparticles. As mentioned in Table 2, biofilm formation was reduced approximately by 40-50% in the presence of TiO2 nanoparticles for both Gram-positive and Gram-negative bacterial strains.
|
S. No |
Bacterial strain |
Biofilm formation |
|
|
Control (without NPs) |
In presence of TiO2 NPs |
||
|
1 |
E. coli |
++++ |
++ |
|
2 |
B. subtilis |
+++++ |
+++ |
Table 2 Effect of TiO2 nanoparticles on bacterial biofilm formation
Representation in table: (++) moderate (++++) strong
In conclusion, the present biotechnological method is capable of producing TiO2 nanoparticles with significant antimicrobial activity. We have used an efficient and eco-friendly approach for rapid synthesis of TiO2 nanoparticles. The bacterium mediated TiO2synthesis exhibits a greater advantage over other conventional techniques in 12-20h. The physicochemical properties of synthesized TiO2 -NPs were investigated by UV-Vis spectroscopy, XRD, TEM, FTIR, Raman, AFM, SEM, PL, and TEM, indicating that TiO2 -NPs crystallize in the anatase phase with smaller size range and it provides a pure form of NPs. The synthesis of TiO2 nanoparticles was confirmed by a colour change of the liquid medium from yellow to intense dark white in colour and it exhibited at maximum absorbance at 324nm play a prominent role in reduction TIP to TiO2 nanoparticles. The XRD studies showed the crystalline nature of nanoparticles. The possibility of protein as stabilizing material in TiO2 nanoparticles is exposed by the FTIR analysis. Nanoparticles with smooth surface morphology and average particle size about 20-30nm were conformed and the anatase TiO2 NPs were formed with the size in the range of 10-30nm that was confirmed by AFM analyses too. These nanoparticles biosynthesis from S. aureus showed excellent antibacterial, antibiofilm, properties against various bacterial strains including E. coli, and Bacillus Subtilis.
These nanoparticles can be further used for the coating purposes in medical devices (e.g. catheters) to control the concerned bacterial infections. The suitability of the TiO2 nanoparticles is because of anti-biofilm activity rather than antibacterial activity, as the pathogenic bacteria grow in/on medical devices by producing biofilm. Presence of biofilm in bacterial colonies shows more resistance towards regular antibiotics and disinfectants used in the hospitals to clean the medical devices such as catheters, pipes used during kidney, other urinary tract infections and ineffective function etc. biomedical applications.
Authors KSL and GKA thank AICTE and Defence Institute of Advanced Technology, Pune, India for providing research Fellowship respectively
Authors declare that there is no conflicts of interest.

©2020 Landage, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.