Journal of
eISSN: 2373-437X


Short Communication Volume 1 Issue 4
Section of Infectious Diseases, Department of Medicine, Georgia Regents University, USA
Correspondence: Jose A Vazquez, Section of Infectious Diseases, Room AE 3029, Department of Medicine, Georgia Regents University, 1120 15th Street, Augusta, GA 30912, USA , Tel 706-723-0105
Received: June 30, 2014 | Published: July 17, 2014
Citation: Manavathu EK, Vazquez JA. Antibacterial activities of stationary phase culture filtrates of Aspergillus fumigates. J Microbiol Exp. 2014;1(4):118-120. DOI: 10.15406/jmen.2014.01.00020
Our previous studies performed for the development of polymicrobial biofilm showed that simultaneous coculturing of Aspergillus fumigatus conidia or sporelings with Pseudomonas aeruginosa resulted in the killing of A. fumigatus cells whereas hyphae older than 12h were recalcitrant to the bacterial fungicidal activity. Since A. fumigatus produces an array of antimicrobial molecules, we examined the antibacterial activity of A. fumigatus culture filtrates on bacterial growth to examine the possible role of such small molecules minimizing the fungicidal activity of P. aeruginosa by spectrophotometric and colony forming unit assays. Culture filtrates collected from 48h stationary phase cultures inhibited the growth of P. aeruginosa and S. aureus, but not that of Candida albicans. Colony forming unit assay of culture filtrate-treated P. aeruginosa cells showed that the effect was bacteriostatic. Culture filtrate incubated in a boiling water bath for 15min retained its antibacterial activity compared to that of the unboiled culture filtrate. These results show that stationary phase liquid cultures of A. fumigatus produce a heat stable bacteriostatic compound(s) in liquid culture that is released into the growth medium inhibits the growth of P. aeruginosa.
Keywords: Culture filtrate, Stationary phase, Antibacterial activity, Aspergillus fumigates, Pseudomonas aeruginosa
Simultaneous static coculturing of A. fumigatus conidia or sporelings with P. aeruginosa cells in vitro results in the initial growth inhibition and eventual death of A. fumigatus cells,1 perhaps elicited by P. aeruginosa produced small diffusible extracellular molecules released into the culture medium.2,3 In contrast, A. fumigatus hyphae grown for 12h or longer from conidia at 35°C in a rich fungal growth medium such as Sabouraud’s dextrose (SD) broth are relatively unaffected by the fungicidal effect of P. aeruginosa culture filtrate.1 The lack of effect of P. aeruginosa culture filtrate on A. fumigatus hyphae could be due to either innate resistance of hyphae to the fungicidal factor(s) and/ or due to the production of an antibacterial factor(s) by the growing A. fumigatus hyphae that either inhibits bacterial growth or blocks the fungicidal activity. Since A. fumigatus is known to produce an array of small molecules4 possessing antimicrobial properties to safeguard its survival, the latter scenario is a likely event in cocultures of P. aeruginosa and A. fumigatus. We therefore investigated the antibacterial activity of A. fumigatus culture filtrates obtained from 24h and 48h cultures of P. aeruginosa.
fumigatus 53470 (a clinical isolate obtained from the Microbiology Laboratory of Henry Ford Hospital, Detroit, MI, USA) was used in this study. Cultures were grown for 24h and 48h in SD broth at 35°C in 6-well Costar cell culture plates. The culture filtrate (CF) was collected and filter-sterilized (0.22µm Millipore filters). For growth inhibition studies, one ml aliquots of the CF supplemented with fresh SD broth was inoculated with 1x107 cells/ ml P. aeruginosa 56402 (a clinical isolate obtained from the Microbiology Laboratory of Henry Ford Hospital in Detroit) in 6-ml polystyrene culture tubes (6 replications) and incubated on a gyratory shaker (170 rpm) at 35°C for 24h. The growth inhibition of P. aeruginosa was determined spectrophotometrically by determining the optical density at 490nm and by colony forming unit (CFU) assay.
The heat stability of the antibacterial factor(s) of the CF was examined after incubating 1ml aliquots in a boiling water bath for 15min followed by the growth inhibition assays. Heat untreated CF and SD broth (1ml aliquots) were used as controls.
The bactericidal effect of the CF was examined by treating 1x107 cells with 1ml CF at 35 °C for 24h. The viability of the treated cells was examined by plating 0.01ml aliquots of the diluted culture on SD agar and determining the CFUs after 24h growth.
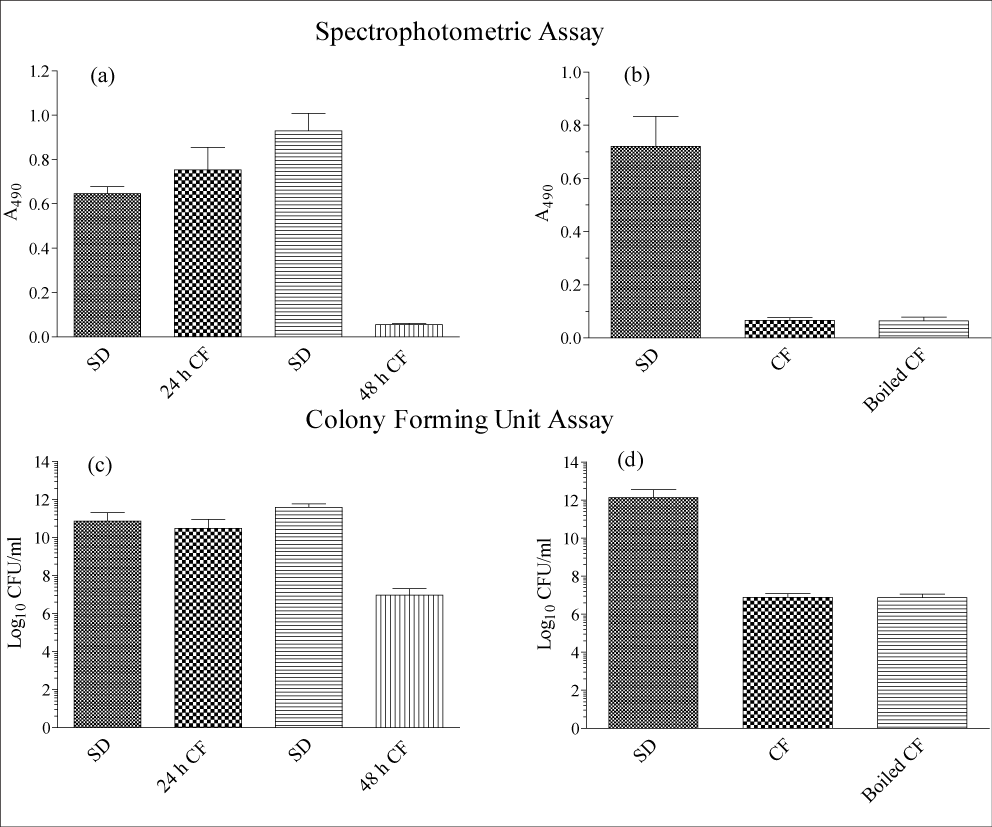
As shown in Figure 1, both spectrophotometric and CFU assays provided similar results. A. fumigatus CF collected from the 48 h stationary phase cultures almost completely inhibited the growth of P. aeruginosa (control: 3.867x1011±2.161x1011 CFU/ml; CF: 9.333x106±1.146x107 CFU/ml; p=0.0071) but that obtained from 24h old culture showed no significant growth inhibition (control: 7.392x1010±1.312x1011CFU/ml; CF: 5.851x1010±2.389x1010 CFU/ml; p=0.540). A CFU assay of P. aeruginosa cells treated with CF showed that the A. fumigatus produced factor was bacteriostatic and no significant reduction of CFUs was obtained for the CF-treated group compared to the initial inoculum whereas the control group showed a CFU increase of 3.5 to 4 logs after 24h incubation at 35°C (Figure 1 panel (c): SD vs. 48h CF and panel (d): SD vs. CF). The CF boiled for 15 min in a water bath retained its antibacterial activity (7.450x106±3.882x106 CFU/ml) compared to the heat untreated culture filtrate (7.717x106±4.490x106 CFU/ml) suggesting that the A. fumigatus produced inhibitory factor is a heat stable molecule(s) (p=0.774). In addition to P. aeruginosa, the stationary phase culture filtrate had similar inhibitory effect on the growth of S. aureus whereas the growth of C. albicans was unaffected.
Mycotoxins, including those produced by A. fumigatus, are low molecular weight organic molecules produced by the fungus to protect itself from predators and competitors in its ecological niche. Among the many toxins produced by A. fumigatus, gliotoxin4 is the most potent and well studied, and it possesses a variety of cytotoxic effects, including triggering apoptosis. We postulated that the CF mediated inhibition of P. aeruginosa is due to gliotoxin or an A. fumigatus produced antibacterial factor(s) perhaps similar to that produced by C. albicans against P. aeruginosa in mixed cultures.5 Bruns et al.,6 and Sekonyela et al.,7 have recently investigated gene expression profiles of young (24h) and mature (48h) A. fumigatus biofilms by Transcriptomics and Proteomics. Not surprisingly, in matured biofilms and liquid shake cultures overall metabolic activity was significantly reduced but energy is channeled to the enhanced expression of genes coding for the biosynthesis of secondary metabolites. In particular, proteins of the gliotoxin secondary metabolite gene cluster were induced in mature biofilm and shake cultures as determined by real-time PCR and HPLC analysis. Our experiments showed that A. fumigatus stationary phase CF inhibited P. aeruginosa growth due to the production a heat stable bacteriostatic compound(s) secreted into the culture medium, and this bacterial growth inhibitory factor(s) may be at least in part responsible for the poor fungicidal effect of P. aeruginosa CF on A. fumigatus hyphae.
Mature biofilms and shake cultures of A. fumigatus is capable of producing an antibacterial factor perhaps similar to gliotoxin to inhibit P. aeruginosa, and this factor may be at least partly responsible for rending protection to A. fumigatus from the fungicidal activity of P. aeruginosa in polymicrobial biofilm.
None.
Authors declare that there is no conflict of interest.

©2014 Manavathu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.