Journal of
eISSN: 2373-437X


Research Article Volume 7 Issue 1
Institute of Microbiology, Beijing Forestry University, China
Correspondence: Ji XiaoHong, Institute of Microbiology, Beijing Forestry University, PO Box 61, Beijing 100083, China
Received: December 19, 2018 | Published: January 10, 2019
Citation: Rui DU, Xiaohong J. A new species of Skeletocutis (Polyporales, Basidiomycota) from Vietnam. J Microbiol Exp. 2019;7(1):20-25. DOI: 10.15406/jmen.2019.07.00234
A new species of Polyporales, now named Skeletocutis vietnamensis, was collected on angiosperm trunks in Vietnam. It is described based on morphological characteristics and molecular evidence. The species has snow white pore surfaces, angular pores mostly 5–7 per mm with entire mouths, a dimitic hyphal structure throughout the trama, generative hyphae in all parts of the basidioma covered by fine crystals, skeletal hyphae slightly inflated in KOH, not agglutinated, and pyriform to ovoid basidiospores measuring 2.8–3.4×1.5–2.1μm. The combination of these characters, spores in particular, separate this species from other species of Skeletocutis. Phylogenetic analysis based on the internal transcribed spacer (ITS) regions indicated that the new species grouped with Skeletocutis species and formed a monophyletic lineage with a strong support (100% BS, 100% BP, 1.00 BPP).
Keywords: Phylogeny, taxonomy, wood, decaying fungi
The genus Skeletocutis Kotl. & Pouzar was established in 1958 and its type species is Skeletocutis amorpha Fr. Kotlába & Pouzar.1 The genus is widely distributed around the world, but the majority of the species known so far are found in the Northern Hemisphere.2‒5 Species in Skeletocutis cause white rot. They mostly have resupinate basidioma although the genus type has pileate or effused-reflexed basidioma. The generative hyphae, at least, partly covered by fine crystals and the tiny basidiospores are the most important characteristics of the genus.3,5 Skeletocutis is phylogenetically close to Tyromyces P. Karst., Ceriporiopsis Domański and Piloporia Niemelä, and they cluster within the Tyromyces clade.6,7 Currently only two species have been described from Southeast Asia, Skeletocutis falsipileata (Corner) T Hatt8 in Malaysia and Skeletocutis bicolor (Lloyd) Ryvarden9 in Singapore. Seven species (Skeletocutis fimbriata Juan Li & YC Dai, S. luteolus BK Cui & YC Dai, S. substellae YC Dai, S. bambusicola LW Zhou & WM, S. inflata BK Cui & YC Dai, S. yunnanensis LS Bian, S. pseudo-odora LF Fan & Jing Si) were described in five provinces in southern China.10‒16 During a survey of lignicolous fungi in Vietnam in October 2017, two specimens were collected growing on angiosperm trunks, and have resupinate basidioma with a distinct cottony sterile margin, relatively small pores, plenty of hyphal pegs, a dimitic hyphal structure, generative hyphae bearing clamp connections and fine, sharp-pointed encrustations especially at the dissepiment edges, bottle-shaped cystidioles and pyriform to ovoid basidiospores. These characters fitted the genus of Skeletocutis, but we could not assign them to a name and so we describe the collections as a new species.
Morphological studies
The studied specimens are deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). The microscopic procedure followed Dai.17 Microscopic measurements were made from slide preparations stained with Cotton Blue, Melzer’s reagent and 5% potassium hydroxide. In the text the following abbreviations were used: KOH=5% potassium hydroxide, IKI=Melzer’s reagent, IKI–=neither amyloid nor dextrinoid, CB = Cotton Blue, CB– =acyanophilous, L=mean spore length (arithmetic average of all spores), W=mean spore width (arithmetic average of all spores), Q=variation in the ratios of L/W between specimens studied, n=number of spores measured from given number of specimens. Color terms follow Petersen.18
Molecular procedures and phylogenetic analyses
The methods of DNA extraction and amplification in this study followed Chen et al.19 A CTAB rapid plant genome extraction kit-DN14 (Aidlab Biotechnologies Co., Ltd, Beijing) was used to extract total genomic DNA from dried specimens of the new collections according to the manufacturer’s instructions with some modifications. The primer pair ITS5 /ITS4 was used for PCR amplifications (primer sequences used in this study were obtained from http://www.biology.duke.edu/fungi/mycolab/primers.htm). The PCR products were sequenced in the Beijing Genomics Institute, China, with the same primers. The newly generated sequences were deposited in GenBank and labeled in Figure 1.
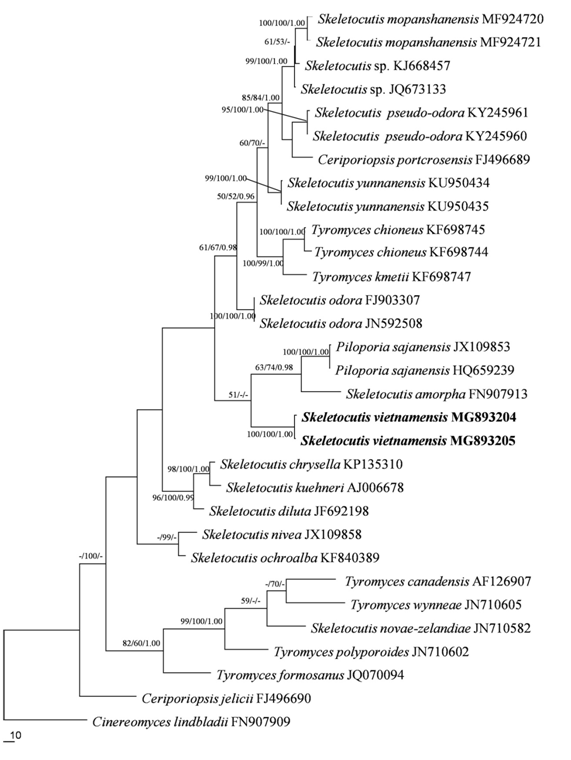
Figure 1 Maximum Parsimony strict consensus tree illustrating the phylogeny of Skeletocutis vietnamensis and related species based on the combined dataset (ITS). Branches are labeled with maximum likelihood bootstrap higher than 50%, parsimony bootstrap proportions higher than 50% and Bayesian posterior probabilities more than 0.95 respectively. The newly sequenced specimens are labeled in boldface.
Besides the newly generated ITS sequences, the current ITS dataset included sequences from all available species of three genera (Piloporia Niemelä, Skeletocutis and Tyromyces P. Karst.) in the Tyromyces clade.7 The ITS sequence of Cinereomyces lindbladii (Berk.) Jülich20 was selected as the outgroup.7 This dataset was aligned using MAFFT 7.11021 with the Q-INS-I opinion.22 Maximum parsimony analysis was applied to the ITS dataset sequences (Table 1).
|
Species |
Specimen no. |
Locality |
GenBank accession no. ITS |
|
Ceriporiopsis jelicii |
H 6002113 |
Finland |
FJ496690 |
|
Ceriporiopsis portcrosensis |
LY 3493 |
France |
FJ496689 |
|
Cinereomyces lindbladii |
H 19911 |
Finland |
FN907909 |
|
Piloporia sajanensis |
Mannine 2733a |
Finland |
HQ659239 |
|
Piloporia sajanensis |
JX 109853 |
Russia |
JX109853 |
|
Skeletocutis amorpha |
Miettinen 11038 |
Finland |
FN907913 |
|
Skeletocutis chrysella |
FD 305 |
USA |
KP135310 |
|
Skeletocutis diluta |
JV 100861 |
USA |
JF692198 |
|
Skeletocutis nivea |
ES 2008 |
Sweden |
JX109858 |
|
Skeletocutis ochroalba |
JK 1208/8 |
Czech Republic |
KF840389 |
|
Skeletocutis kuehneri |
Sample 115 |
AJ006678 |
|
|
Skeletocutis mopanshanensis |
MF 924720 |
China |
MF924720 |
|
Skeletocutis mopanshanensis |
CLZhao 1184 |
China |
MF924721 |
|
Skeletocutis novae-zelandiae |
X 489 |
New Zealand |
JN710582 |
|
Skeletocutis odora |
B 40 |
Latvia |
FJ903307 |
|
Skeletocutis odora |
JV 1007-7 |
Czech Republic |
JN592508 |
|
Skeletocutis pseudo-odora |
KY 245961 |
China |
KY245961 |
|
Skeletocutis pseudo-odora |
KY 245960 |
China |
KY245960 |
|
Skeletocutis sp. |
KUC 20121109-07 |
South Korea |
KJ668457 |
|
Skeletocutis sp. |
DLL 2010-112 |
— |
JQ673133 |
|
Skeletocutis vietnamensis |
Dai 18378 |
Vietnam |
MG893204 |
|
Skeletocutis vietnamensis |
Dai 18374 |
Vietnam |
MG893205 |
|
Skeletocutis yunnanensis |
Dai 15709 |
China |
KU950434 |
|
Skeletocutis yunnanensis |
Dai 15712 |
China |
KU950435 |
|
Tyromyces canadensis |
Pentill 1321 |
Finland |
AF126907 |
|
Tyromyces chioneus |
Cui 10225 |
China |
KF698745 |
|
Tyromyces chioneus |
Cui 7191 |
China |
KF698744 |
|
Tyromyces formosanus |
MYA-262 |
— |
JQ070094 |
|
Tyromyces kmetii |
Dai 12403 |
China |
KF698747 |
|
Tyromyces polyporoides |
X 510 |
Venezuela |
JN710602 |
|
Tyromyces wynneae |
X 1217 |
Denmark |
JN710605 |
Table 1 A list of species, specimens and GenBank accession numbers of sequences used in this study. New sequences are shown in bold.
Phylogeny
The ITS dataset, including 31 sequences, resulted in an alignment with 666 characters, of which 246 characters are constant, 88 variable characters are parsimony-uninformative and 332 characters are parsimony-informative. MP analysis yielded seven equally parsimonious trees (TL=1361, CI=0.554, HI=0.446, RI=0.665, RC=0.369). Best model for the ITS dataset estimated and applied in the Bayesian analysis: GTR+I+G. Bayesian analysis and ML analysis resulted in a similar topology as MP analysis, with an average standard deviation of split frequencies=0.009615 (BI). The new species clustered in the Tyromyces clade and formed a monophyletic lineage with a high support (100% BS, 100% BP, 1.00 BPP).
Taxonomy
Skeletocutis vietnamensis Rui Du & XH Ji, sp. nov. (Figure 2 & Figure 3)
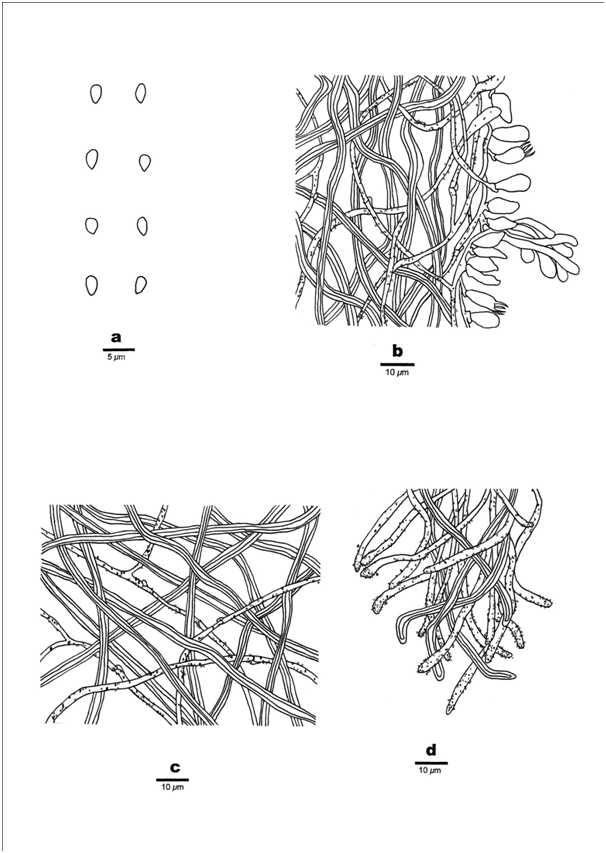
Figure 3 Microscopic structures of Skeletocutis vietnamensis (Holotype). a) Basidiospores, b) Section from tube trama, c) Hyphae from subiculum, d) Section of dissepiment edge. Scale bars: a) 5μm; b–d) 10μm.
MycoBank number MB 824183;
Holotype –Vietnam. Lam Dong Province, Lac Duong District, Bidoup Nui Ba Park, on rotten angiosperm trunk, 15 October 2017, Dai 18378 (Holotype in BJFC 0024888).
Etymology–Vietnamensis (Lat.): refers to the locality of the type species in Vietnam.
Fruiting body – Basidioma perennial, resupinate, very difficult to separate from substrate, soft leathery, and without odour or taste when fresh, becoming hard corky upon drying, up to 7.5cm long, 5cm wide, and 2.4mm thick at center. Pore surface snow white when fresh, honey yellow when bruised, white to cream upon drying, bruised part becoming buff-yellow when dry; sterile margin distinct when juvenile, byssoid to cottony, consistently white, up to 1.5mm, becoming narrow to almost lacking when mature; pores angular, freely arranged, mostly 5–7 per mm; dissepiments thin, entire. Subiculum white, hard corky, up to 0.4mm thick. Tubes darker than poroid surface, hard corky, up to 2mm long.
Hyphal Structure–Hyphal system dimitic, generative hyphae with clamp connections, hyaline, thin-walled, dominant at dissepiment edge; skeletal hyphae thick-walled with a narrow lumen to subsolid; IKI–, CB–, slightly inflated in KOH.
Context –Generative hyphae frequent, hyaline, thin- to slightly thick-walled, rarely branched and bearing fine crystals, 1.5–2.5μm in diameter; skeletal hyphae in trama dominant, thick-walled, flexuous, unbranched, interwoven, 2–3μm in diameter.
Tubes–Generative hyphae frequent, thin- to slightly thick-walled, occasionally branched, usually covered by fine, sharply pointed encrustations, especially at dissepiment edge, 1.5–2.5μm in diameter, and occasionally branched; Skeletal hyphae dominant, thick-walled with a narrow lumen to subsolid, unbranched, subparallel along the tubes, not agglutinated, 2–3μm in diameter. Dissepiment edge dimitic with smooth skeletal hyphae, and dominant winding, encrusted generative hyphae. Cystidia absent, cystidioles abundant, bottle-shaped, with a conical apex, almost the size of the basidia, 8–12×3–4.5μm; basidia infrequent, broadly clavate with a basal clamp connection and four sterigmata, 10–11×3–4.5μm; basidioles in shape similar to basidia, but slightly smaller. Hyphal pegs glomeratus and frequent.
Spores–Basidiospores mostly pyriform to ovoid, hyaline, thin-walled, smooth, CB–, IKI–, (2.6–)2.8–3.4×1.5–2.1(–2.2)μm, L=2.87μm, W=1.72μm, Q=1.67 (n=10/1).
Notes–Skeletocutis vietnamensis is characterized by resupinate, snow white pores when fresh but white to cream when dry, distinct byssoid to cottony sterile margin, small pores 5–7 per mm with entire dissepiments, a dimitic hyphal structure with skeletal hyphae in all parts of the basidioma, the presence of hyphal pegs, all generative hyphae covered by fine crystals, skeletal hyphae slightly inflated in KOH, pyriform to ovoid basidiospores measuring 2.8–3.4×1.5–2.1μm and growing on angiosperm wood. Microscopically S. vietnamensis is similar to S. krawtzewii (Pilát) Kotl. & Pouzar. But S. vietnamensis can be distinguished by having pyriform to ovoid spores and bottle-shaped cystidioles with a conical apex.
Additional specimen examined (paratype)–Vietnam. Lam Dong Province, Lac Duong District, Bidoup Nui Ba Park, on fallen angiosperm trunk, 15 October 2017, Dai 18374 (BJFC 024887).
Phylogenetically Skeletocutis is a diverse genus, with Tyromyces and Piloporia in the same clade; Tyromyces is deeply embedded in a big clade which is mainly composed of Skeletocutis species.27 Tyromyces fruit bodies are annual, resupinate to pileate, with a pileus that is finely tomentose to smooth, usually white, more rarely bluish, reddish to brownish, pore surface white to cream, rarely greyish brown or reddish by drying, pores regular, round to angular, context or subiculum white to light brown when dry, consistency soft when fresh, mostly fragile and light weighted when dry. The hyphal system is usually monomitic, rarely dimitic, generative hyphae are hyaline, normally with clamps and lack crystals, cystidia are absent or present, when dimitic either with rather few binding hyphae or hyaline skeletal hyphae, spores are allantoid, cylindrical to ellipsoid, hyaline, smooth, and thin-walled.5,28 Piloporia basidioma are pileate, effused-reflexed to resupinate with a tomentose dark brown upper surface, a pore surface that is whitish to cork-colored with concolorous tubes; a duplex context with a black line separating the lower cork-colored part from the upper rusty brown part; a dimitic hyphal system; generative hyphae with clamps; skeletal hyphae hyaline to brown in upper part of context and finely encrusted in the dissepiments; absence of cystidia, and basidiospores that are allantoid, hyaline, and thin-walled.29
Skeletocutis basidioma are pileate, effused-reflexed to resupinate with a dark brown tomentose upper surface, a whitish to cork-colored pore surface with concolorous tubes, a duplex context with a black line separating the lower cork-colored part from the upper rusty brown part; a dimitic hyphal system; generative hyphae with clamps; skeletal hyphae hyaline to brown in upper part of context and finely encrusted in the dissepiments and absence of cystidia. The generative hyphae are at least partly covered by fine crystals and the basidiospores are allantoid, hyaline and thin-walled.3,29 The new species we describe fits the characteristics of Skeletocutis, so we have named it S. vietnamensis. Microscopically S. vietnamensis is very similar to S. krawtzewii, and both species have frequent hyphal pegs. However, S. krawtzewii has rounded spores, amygdaliform cystidioles, and a monomitic hyphal structure which only contain generative hyphae at the dissepiment edge.3,30 In contrast, S. vietnamensis has pyriform to ovoid spores, bottle-shaped cystidioles with a conical apex, and a dissepiment edge with skeletal hyphae. Phylogenetically, S.vietnamensis is related to S. amorpha and Piloporia sajanensis (Parmasto) Niemelä,31 but S. amorpha can be distinguished by the pileate or effused-reflexed basidioma and the cartilaginous, pinkish buff to reddish orange pore surface.1 Piloporia sajanensis differs from S. vietnamensis by the pileate basidioma, duplex context, absence of hyphal pegs and allantoid basidiospores measuring 3.5–4.0×0.8–1μm.31
We express our gratitude to Prof. Yu-Cheng Dai (BJFC, China) who allowed us to study his specimens. The research was supported by the Fundamental Research Funds for the Central Universities (Project No. BLX201622).
Authors declare that there is no conflict of interest.

©2019 Rui, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.