Journal of
eISSN: 2373-437X


Mini Review Volume 5 Issue 4
Institute of Biochemistry and Molecular Biology, National Yang Ming University, Taiwan
Correspondence: Gwo Chyuan Shaw, Institute of Biochemistry and Molecular Biology, School of Life Sciences, National Yang Ming University, Taipei 112, Taiwan, Tel 886-2-28267127, Fax 886-2-28264843
Received: June 01, 2017 | Published: July 31, 2017
Citation: Shaw GC (2017) A New Physiological Role for CcpA in Adaptation of Bacillus Subtilis to Sugar-Induced Osmotic Stress. J Microbiol Exp 5(4): 00155. DOI: 10.15406/jmen.2017.05.00155
The model Gram-positive bacterium Bacillus subtilis is liable to be exposed to high-salinity environments in its natural habitats and is often used in fermentation with high concentrations of glucose or other sugars. High salinity or high concentrations of sugars can cause osmotic stress to B. subtilis. Past researches regarding osmoadaptation of B. subtilis were mainly focused on responses to salt-induced osmotic stress. There was little or no mention about how B. subtilis cells responded to sugar-induced osmotic stress. The catabolite control protein (CcpA) is known to be a global transcriptional regulator that mediates glucose repression of many catabolic genes and activation of genes involved in excretion of excess carbon in various Bacillus species. However, the physiological significance for CcpA-mediated sugar activation of degU, gltAB, opuA, opuE, proHJ and the ilvB operon remained poorly defined. Here based on the results from the literature search, it is now proposed that CcpA-mediated sugar activation of these osmoadaptive genes may facilitate the adaptation of B. subtilis to sugar-induced osmotic stress. This finding could add a new physiological role to CcpA in B. subtilis and probably its close relatives. As to biotechnological application, construction of B. subtilis strains that could over-express CcpA might potentially enhance their abilities to withstand higher concentrations of sugars for producing higher yields of various economically effective fermentation products.
Keywords: Bacillus subtilis, catabolite control protein, ccpa, osmoadaptation, sugar induced osmotic stress
CcpA, catabolite control protein; PTS, phosphor transferase system
The model Gram-positive bacterium Bacillus subtilis is liable to be exposed to high-salinity environments in its natural habitats and is often used in fermentation with high concentrations of glucose or other sugars. High salinity or high concentrations of sugars can cause osmotic stress to B. subtilis.1 The salt-induced osmotic stress can activate signalling pathways to induce expression of genes for biosynthesis or uptake of osmotically compatible solutes, thus protecting B. subtilis cells against the salt stress. Glycine betaine and proline are two important compatible solutes that can be utilized by B. subtilis to cope with salt stress.2 The salt-inducible opuA operon encodes an ABC transporter that is involved in the uptake of glycine betaine for defense of B. subtilis against salt stress.3 OpuE is a proline uptake transporter that can be induced by high salinity for osmotolerance.4 The salt-inducible proHJ operon encodes enzymes involved in biosynthesis of proline for osmotic adaptation.1 The two-component signal transduction system DegSU can sense high salinity and be induced by salt stress. The response regulator DegU is a positive regulator of the osmotic response. Mutation of degU confers an osmosensitive phenotype to B. subtilis.5,6 Past researches regarding osmoprotection of B. subtilis were mainly focused on responses to salt-induced osmotic stress. There was little or no mention about how B. subtilis cells responded to sugar-induced osmotic stress. The catabolite control protein (CcpA) is known to be a global transcriptional regulator that mediates glucose repression of many catabolic genes and activation of genes involved in excretion of excess carbon in various Bacillus species.7 A literature search has revealed that glucose can also CcpA-dependently activate expression of the degU gene, the gltAB operon, the ilvB operon, the opuA operon and the opuE gene.8–11 The ilvB operon of B. subtilis encodes enzymes involved in biosynthesis of the branched-chain amino acid isoleucine. Salt-induced accumulation of isoleucine is known to play an important role in tolerance of plants to salt stress.12 The gltAB operon of B. subtilis encodes glutamate synthase. Glutamate is a precursor of proline biosynthesis and it per se can function as an osmotically compatible solute.13 A previous report has shown that sucrose and lactose can induce proHJ expression.1 Glucose can also activate proHJ expression via CcpA (CJ Lin and GC Shaw, unpublished observations). The biological significance for CcpA-mediated glucose activation of the gltAB operon or the ilvB operon was previously suggested to be a link between carbon and nitrogen metabolism.8,14 The physiological significance for CcpA-mediated glucose activation of degU was postulated to be relevant with consumption of acetyl-coenzyme A during polyketide synthesi.11 However, the biological significance for CcpA-mediated sugar activation of degU, gltAB, opuA, opuE, proHJ and the ilvB operon remained poorly defined. In Escherichia coli, ProP is a member transporter for uptake of proline and other osmoprotectants. ProP is also involved in sensing the osmotic stress caused by high salinity.15 In B. subtilis, the sensor for perception of the osmotic stress caused by high salinity or high concentrations of sugars has not yet been identified. Nevertheless, it is known that glucose is transported into B. subtilis cells by the glucose-specific phosphoenolpyruvate: sugar phosphotransferase system (PTS) encoded by the ptsGHI operon.16 EIICBA is the gene product of ptsG and is a membrane transporter responsible for glucose transport and phosphorylation. Expression of the ptsGHI operon is known to be glucose-inducible.16
Here based on the results from the literature search, it is now proposed that sugar (including glucose and probably fructose, sucrose or lactose) activation of these osmoadaptive genes via CcpA may facilitate the adaptation of B. subtilis to sugar-induced osmotic stress. EIICBA may possibly be involved in sensing the osmotic stress caused by glucose and transducing the signal to CcpA via HPr and/or Crh17 to activate osmoadaptive genes for adaptation of B. subtilis to glucose-induced osmotic stress (Figure 1). This finding could add a new physiological role to CcpA in B. subtilis and probably its close relatives. As to biotechnological application, construction of B. subtilis strains that could over-express CcpA might potentially enhance their abilities to withstand higher concentrations of sugars for producing higher yields of various economically effective fermentation products.
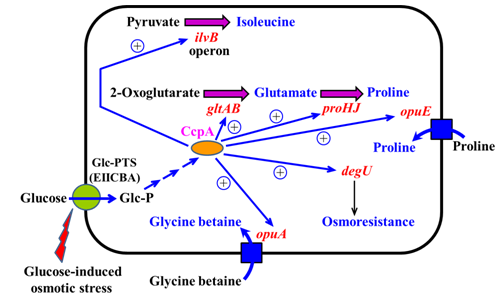
Figure 1 A model for the regulatory network of CcpA-mediated glucose activation of osmoadaptive genes for adaptation of B. subtilis to glucose-induced osmotic stress. Osmoadaptive genes encode proteins responsible for biosynthesis or uptake of osmoprotectants such as glutamate, glycine betaine, isoleucine and proline. CcpA-mediated glucose activation of degU expression can confer protection of B. subtilis against glucose-induced osmotic stress. The glucose-inducible membrane transporter EIICBA may play a role in sensing the osmotic stress caused by high concentrations of glucose and transducing the signal to CcpA via HPr and/or Crh for activation of osmoadaptive genes.
Abbreviations: Glc-P, phosphorylated glucose; Glc-PTS, glucose-specific phosphoenolpyruvate: sugar phosphotransferase system
This work was supported by grant MOST 104-2311-B-010-006-MY2 from the Ministry of Science and Technology of the Republic of China (Taiwan).
No conflict of interest was declared.

©2017 Shaw. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.