Journal of
eISSN: 2373-437X


Polycyclic aromatic hydrocarbons (PAHs) are aromatic hydrocarbons consisting of two or more fused aromatic benzene rings. They are formed during the incomplete combustion of organic substances. They enter the environment and are released to air, soil, water and foods. Some PAHs have been shown to have toxicological, carcinogenic and mutagenic effects on animals and humans. Persistence of some types of lactic acid bacteria (S. thermophilus, L. bulgaricus and B. bifidium) in the presence of different levels (0.01, 0.10 and 0.20µg/ml MRS medium) of PAHs were studied during the incubation periods for (24, 48, 72, 96 and 120 hr) at 37 ˚C. The growth rate of the studied strains noticeably affected for presence of PAHs according to bacterial species and the PAHs concentrations. After 24hr of incubation period, the relative reduction of S. thermophilus, L. bulgaricus and B. bifidium growth rate in MRS media broth containing 0.01µg PAHs/ml were ranged between (2.33-9.43%) increased to (11.63-32.08%) with 0.10µg PAHs/ml and to (23.26-38.1%) with 0.20µg PAHs/ml. At the end of the incubation period (120hr), the relative increase of growth rate was observed by S. thermophilus and relative reduction with the other two strains. The data proved that the effect of the lowest concentrations of PAHs on the strains not impressive, but PAHs might have an impact if concentrations are higher.
Keywords: Lactic acid bacteria; Polycyclic aromatic hydrocarbons; Total viable counts
Polycyclic aromatic hydrocarbons (PAHs) are aromatic hydrocarbons consisting of two or more fused aromatic benzene rings. They are lipophilic in nature and not readily soluble in water [1]. These compounds tend to partition in the fatty tissues once organisms ingest them. The increase in the electrochemical stability is associated with an increase in the number of benzene rings and angularity of a PAH molecule [1,2]. The high molecular weight (HMW) PAHs are more persistent and recalcitrant than the low molecular weight (LMW) PAHs [3]. The stability and distribution of the PAHs in the natural environment is influenced by the configuration of the aromatic rings [4] and physico-chemical properties [5].
Polycyclic aromatic hydrocarbons (PAHs) are formed during the incomplete combustion or high-temperature pyrolysis of coal, oil, gas, wood, fossil fuels, garbage or other organic substances, such as tobacco and charbroiled meat [6]. The quantity and composition of PAHs produced are closely related to the reaction conditions, temperature and amount of air and therefore, may vary considerably [7]. These compounds comprise the largest class of known chemical carcinogens and have been detected in the environment especially in air, water, soil and foods [8,9]. They enter also to the environment mostly as releases to air from volcanoes, forest fires, and residential wood burning, cigarette smoke, asphalt roads, coal, coal tar, agricultural burning, municipal, industrial waste incineration, hazardous waste sites and exhaust from automobiles and trucks. PAHs are absorbed through the gastrointestinal tract when ingested [2]. After being absorbed, they are distributed throughout the body, including all internal organs [2]. Some PAHs have been shown to have genotoxic effects both in vivo in rodents and in vitro in mammalian (including human) cell lines and prokaryotes [2]. On the other hand, some PAHs do not appear to be genotoxic.
Foods may be contaminated through the different routes, which include following: direct deposition of PAHs from the atmosphere as environmental contaminants and contaminated packing materials and production of PAHs during the thermal processing of foods, e.g. drying, baking, grilling and smoking [10]. PAHs are found in substantial quantities in some foods, depending on the mode of cooking, preservation and storage, and are detected in a wide range of meats (2.611 mg/kg), fishes (1.4811- 2.6782 mg/kg), vegetables (6.196 - 8.977 mg/kg) and fruits (2.334-2.867 mg/kg) [8,9].
The use of beneficial microorganisms in the food sector has a long tradition, namely lactic acid bacteria, in fermentation processes. They are widely used in the manufacture of fermented food and are among the best studied microorganisms. Detailed knowledge of a number of physiological traits has opened novel potential applications for these organisms in the food industry, while other traits might be specifically towards beneficial for human health [11]. Also the main examples encompass the lactic acid bacteria is their ability to synthesize antimicrobial substances. Such properties may allow substitution of natural compounds for chemical food preservative [12].
The term “microbial growth” is related to the multiplication of the microbial population, which might be affected by presence of different pollutants [13-15]. Studies made by Kohler et al. [16] and Jagnow et al. [17] demonstrated that the physiological state of the bacteria influences the biodegradation process. The effect of these contaminants on the beneficial bacteria, such as lactic acid bacteria could be lead to some technological problems in the sensory properties and quality of the resultant products.
The aim of this study presents more detailed information concerning the growth rate of some type’s lactic acid bacteria (dairy and fermented foods starter) in media containing PAHs as contaminants.
Materials
Chemicals
Polycyclic aromatic hydrocarbons (PAHs) reference standards: A mixture (16 compounds) of PAHs reference standards containing acetaphthene, acenaphthylene, anthracene, benzo(a)anthracene, benzo(a)pyrene, benzo(b)fluoranthene, benzo(g,h,i)perylene, chrysene, dibenz(a,h)anthracene, fluoranthene, fluorene, indeno(1,2,3,-cd)pyrene, naphthalene, phenanthrene, pyrene and 2-bromonaphthalene was purchased from Supelco company (Supleco Park, Bellefonte, PA, U.S.A.). The chemical structure and formula indices of these compounds are mentioned in Table 1.
PAHs |
Molecular Weight |
Chemical Formula |
|
1 |
Naphthalene |
128 |
C10H2 |
2 |
Acenaphthylene |
152 |
C12H8 |
3 |
2.Bromonaphthalene |
206 |
C10H7Br |
4 |
Acenaphthene |
154 |
C12H10 |
5 |
Fluorene |
166 |
C13H10 |
6 |
Anthracene |
178 |
C14H10 |
7 |
Phenanthrene |
178 |
C14H10 |
8 |
Pyrene |
202 |
C16H10 |
9 |
Fluoranthene |
202 |
C16H10 |
10 |
Chrysene |
228 |
C18H12 |
11 |
Benzo(a)anthracene |
228 |
C18H12 |
12 |
Benzo(k)fluoranthene |
252 |
C20H12 |
13 |
Benzo(a)pyrene |
252 |
C20H12 |
14 |
Benzo(ghi)perylene |
276 |
C22H12 |
15 |
Dibenz(a,h)anthracene |
278 |
C22H14 |
16 |
Indeno(1,2.3-cd)pyrene |
276 |
C22H12 |
Table 1: Chemical formula of polycyclic aromatic hydrocarbons (PAHs).
Bacterial cultures: Pure strains belonging to Streptococcus thermophilus (S. thermophilus), Lactobacillus bulgaricus (L. bulgaricus) and Bifidobacterium bifidium (B. bifidium) were obtained from Cairo Microbiological Research Center, Cairo MIRCEN, Faculty of Agriculture, Ain-Shams University, Egypt. The strains were stored at -18 ˚C until utilized. An 18 hours old actively growing culture in broth was used to inoculate the sterile media.
Medium: De Man-Rogosa-Sharpe (MRS) broth was obtained from Oxoid, England for propagation and enumeration of all strains studied (S. thermophilus, L. bulgaricus and B. bifidium).
Conventional chemicals and solvents: Methylene chloride (dichloromethane) (Chromatography grade) was obtained from Merck (Darmstadt, Germany). Water was distilled using Milli Q water purification for medium preparation.
Methods
1-Lactic acid bacterial counts: Sterilized (at 121 ˚C/15 min) MRS liquid medium was prepared according to Man et al. [18] Three liters of medium broth was prepared and divided into three flasks (one liter media of each). The first flask inoculated by 1% B. bifidium, the second flask inoculated by 1% S. thermophilus and the third flask inoculated by L. bulgaricus. Each flask divided into five flasks each of them containing 200ml media. The three first flasks were contaminated by PAHs at levels of 0.01, 0.1, 0.2µg/ml medium broth, the other two flasks, one was control with 5µl dichloromethane/ml medium (representing the actual solvent used in dissolving the different concentrations of PAHs) broth and the other flask was control without solvent. Each flask (200ml) was divided in test tubes (9.9ml). All the test tubes were incubated at 37 ˚C and the viable counts were determined after, zero, 24, 48, 72, 96 and 120hr intervals as colony forming units (CFU). Mean of CFU represent the count of three replicate from each dilution (three replicate of dilution). In addition, the experimental repeated three times and mean of CFU were recorded.
2-Statistical analysis: The data were statistically analyzed by analysis of variance and least significant difference (L.S.D) at 0.05 levels according to the method described by Snedecor & Cochran [19].
Persistence of some lactic acid bacteria (LAB) as affected by PAHs
The presence of dichloromethane up to 5µl per 1 ml MRS medium broth (as control) did not affect the total viable counts of tested strains (S. thermophilus, L. bulgaricus and B. bifidium). The influence of different concentrations of PAHs (0.01, 0.10 and 0.20µg/ml) on activity of the three studied LAB during the incubation period for 120hr are presented in Tables 2 to 7 and illustrated in Figures 1 to 3. These results indicate that growth rate of all strains under investigation were affected due to presence of PAHs. However, it could be revealing that the effect of PAHs depends on bacterial species and the PAHs concentrations as follows:
Streptococcus thermophilus (S. thermophilus): The growth rate of S. thermophilus with presence different concentrations of PAHs (0.01, 0.10 and 0.20µg/ml) was affected during the first 24 hr (lag-phase) compared with untreated sample (Table 2,3 and Figure 1&2). However, with progressing the incubation period, growth rate rapidly increases in both control and PAHs treatments samples. Nevertheless, the microorganism rapidly adapted with presence of such PAHs and grow fast to accomplish higher population after 120hr than the control (96hr).
PAHs concentrations (µg/ml) |
|||||||||
Incubation period (hr) |
Control** |
0.01 |
0.1 |
0.2 |
LSD at 5% |
||||
Count |
Log*±SD |
Count |
Log*±SD |
Count |
Log*±SD |
Count |
Log*±SD |
||
24 |
53x107 |
12.07a±1.99 |
48x107 |
11.77b±1.99 |
36x107 |
10.89c±1.05 |
33x107 |
10.63d±1.03 |
4.02 |
48 |
74x108 |
14.95a±1.10 |
72x108 |
14.86b±1.91 |
61x108 |
14.28c±1.10 |
54x108 |
13.86d±0.01 |
2.55 |
72 |
86x108 |
15.48a±1.99 |
77x108 |
15.09b±1.99 |
70x108 |
14.76c±1.10 |
66x108 |
14.56d±1.10 |
2.94 |
96 |
88x108 |
15.56a±1.10 |
78x108 |
15.14b±0.01 |
75x108 |
15.00c±1.10 |
70x108 |
14.76d±1.10 |
1.8 |
120 |
63x108 |
14.39c±1.10 |
77x108 |
15.09a±1.91 |
74x108 |
14.95b±1.10 |
74x108 |
14.95b±1.10 |
2.55 |
Table 2: Growth counts of S. thermophilus in MRS medium broth containing different levels of PAHs during incubation at 37 ˚C for 120hr.
*Log count bacteria±Standard Deviation (SD).
**Average of data obtained from MRS media broth containing 5µl dichloromethane/1ml MRS medium broth
- Log10 initial count (zero time)=0.78.
-All values are means of triplicate determination.
-Means of log-count column with different letters are significantly different (p<0.05).
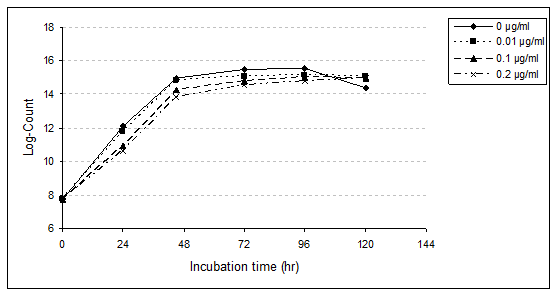
Data showed that S. thermophilus in MRS medium broth control, as well as 0.01 and 0.10µg/ml treated samples achieved its highest viable count and reached the stationary phase after incubation period for 96 hr at 37 ˚C (log counts were 15.56, 15.14 and 15.00). However, in the presence of PAHs at level 0.20µg/ml, the same phase was attained after 120hr (log count was 14.95).
These results indicate the inhibitory effect of PAHs (Table 3) on the viable count of the strain during the incubation period for 24, 48 and 96hr. The relative reduction of S. thermophilus growth in MRS medium broth containing 0.01, 0.10 and 0.20µg/ml were 9.43, 32.08 and 37.74% after 24hr; 2.70, 17.57 and 27.03% after 48hr; 10.47, 10.47 and 23.26% after 72hr and 11.36, 14.77 and 20.45% after 96hr, respectively. However, the viable counts of bacteria in treated samples were higher than the untreated sample at the end of incubation time (120hr). The relative increases were 22.22, 17.46 and 17.46 % with treated samples by 0.01, 0.10 and 0.20µg/ml, respectively. The present results proved that viable count of bacteria decreased with increasing the level of PAHs especially at the first time of incubation due to bacteriostatic and bactericidal effects.
PAHs concentrations (µg/ml) |
|||
Incubation period (hrs) |
0.01 |
0.1 |
0.2 |
24 |
9.43 |
32.08 |
37.74 |
48 |
2.7 |
17.57 |
27.03 |
72 |
10.47 |
10.47 |
23.26 |
96 |
11.36 |
14.77 |
20.45 |
120 |
22.22* |
17.46* |
17.46* |
Table 3: Relative reduction or relative increase of S. thermophilus counts in MRS media broth as affected by the presence of different levels of PAH.
*Relative increase.
Statistical analysis proved the significant difference (p<0.01) between the used concentrations and the viable counts of the strain as the concentration of PAHs and the incubation period. The level of 0.20µg/ml caused greatest harmful effect, followed by 0.10 and 0.01µg/ml within the first 24 hr of incubation. This result confirmed by statistical analysis, which highly significant differences were observed with the higher concentration (0.20µg/ml) compared with the untreated sample. However, slightly significant differences were recorded with lower concentrations (0.01 and 0.10µg/ml). The same pattern was observed during the incubation period up till 96hr. At the end of the incubation period, statistical analysis proved that slightly or insignificant difference was detected with the three treatment samples. Besides, highly significant differences were reported between the treatment samples and un-treatment sample (control).
No available data concerning the effect of PAHs on the viable counts of S. thermophilus. However, the same pattern was observed with the effect of some pesticides (represent the same group of PAHs, i.e. persistence organic pollutants) on this strain. Yankov & Peeva [20] reported that the bacteriostatic and bactericidal effects were observed at high levels of DDT being 1000 and 10.000 ppm, respectively. Similar results were recorded by Gajduskova & Lat [21] with malathion. On the other hand, Abou-Arab [20] reported that 50-200ppm of fenvalerate, malathion and DDT caused harmful effects on S. thermophilus. In addition, Abou-Arab [15] proved that the presence of 0.01-3.0ppm of β-BHC, 0.01-1.00ppm lindane and 0.02-2.00ppm ρ.ρ-DDT have no significant effect on growth rate of the strain.
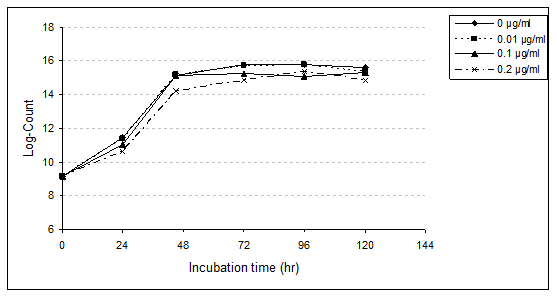
Lactobacillus bulgaricus (L. bulgaricus): Enumeration of L. bulgaricus in MRS medium broth with or without PAHs was studied (Table 4, 5 and Fig. 2). Presence of the three levels of PAHs (0.01, 0.10 and 0.20 µg/ml) was an enhanced growth rate of L. bulgaricus, achieving equal or higher counts than the control. At the first 24hr of incubation (lag phase) log counts of bacteria were 11.43, 11.36, 11.06 and 10.63 with control, 0.01, 0.10 and 0.20µg/ml, respectively. These results indicate a slight reduction (2.33%) with PAHs at the lowest level (0.01µg/ml). The relative reduction increased to 11.63% with 0.10µg/ml as well as 23.26% with 0.20µg/ml (Table 5). After 48hr of incubation presence of 0.01 and 0.10µg/ml enhanced growth rate of L. bulgaricus, achieving equal or higher counts the control.
PAHs concentrations (µg/ml) |
|||||||||
Incubation period (hr) |
Control** |
0.01 |
0.1 |
0.2 |
LSD at 5% |
||||
Count |
Log*±SD |
Count |
Log*±SD |
Count |
Log*±SD |
Count |
Log*±SD |
||
24 |
43x107 |
11.43a±1.10 |
42x107 |
11.36b±1.10 |
38x107 |
11.06c±1.10 |
33x107 |
10.63d±1.03 |
1.8 |
48 |
78x108 |
15.14b±1.10 |
79x108 |
15.18a±1.10 |
78x108 |
15.14b±1.10 |
60x108 |
14.22c±1.99 |
1.8 |
72 |
94x108 |
15.79a±1.10 |
92x108 |
15.71b±1.10 |
81x108 |
15.27c±1.10 |
71x108 |
14.81d±1.03 |
1.8 |
96 |
93x108 |
15.75b±1.10 |
94x108 |
15.79a±1.24 |
91x108 |
15.07d±1.24 |
83x108 |
15.35c±1.10 |
2.08 |
120 |
89x108 |
15.6a±1.10 |
84x108 |
15.39b±1.23 |
82x108 |
15.3c±1.10 |
72x108 |
14.86d±1.10 |
1.8 |
Table 4: Growth counts of L. bulgaricus in MRS media broth containing different levels of PAHs during incubation at 37 ˚C for 120hr.
*Log count bacteria±Standard Deviation (SD).
**Average of data obtained from MRS media broth containing 5µl dichloromethane/1ml MRS medium broth.
- Log10 initial count (zero time)=9.19.
-All values are means of triplicate determination.
-Means of log-count column with different letters are significantly different (p<0.05).
PAHs concentrations (µg/ml) |
|||
Incubation period (hr) |
0.01 |
0.1 |
0.2 |
24 |
2.33 |
11.63 |
23.26 |
48 |
1.28* |
-- |
23.08 |
72 |
2.13 |
13.83 |
24.47 |
96 |
1.08* |
2.15 |
10.75 |
120 |
5.62 |
7.87 |
19.1 |
Table 6: Relative reduction or relative increase of L. bulgaricus counts in MRS medium broth as affected by presence of different levels of PAH.
*Relative increase.
However, at 0.20µg/ml PAHs decreased the viable count by 23.08%. With progressing the incubation period, the growth rate of strain rapidly increased. The highest viable counts of bacteria were after 72hr in control, 96hr with a treated sample by 0.01µg/ml, as well as 120hr for both treated samples of 0.10 and 0.20µg/ml. However, the relative reduction of bacterial counts was observed with the different concentrations of PAHs. This reduction, increased with the higher concentrations. After 120hr (end of the incubation period), the relative reduction were 5.62, 7.87 and 19.10% in 0.01, 0.10 and 0.20µg/ml of PAHs, respectively.
Statistical analysis confirmed the highly significant influences (p<0.01) of the higher concentrations (0.10 and 0.20µg/ml). However, insignificant differences (p<0.01) of the lowest level of PAHs (0.01µg/ml) in this respect. Reviewing the aforementioned results, it could be concluded that PAHs at higher levels caused the greatest harmful effects on L. bulgaricus counts.
No available data concerning the effect of PAHs on L. bulgaricus. However, similar results reported by Abou-Arab [22] with pesticides. The author found a relative reduction of L. bulgaricus counts with high concentrations (50-200 ppm) of DDT, malathion and fenvalerate during the incubation period. On the other hand, Abou-Arab [15] reveals that the presence of low concentrations (0.01-0.02 ppm) of β-BHC and ρ.ρ-DDT triggered acid production of L. bulgaricus. However, such enhancement occurred in the presence of lindane at concentrations up to 1.0 ppm.
Bifidobacterium bifidum (B. bifidum): B. bifidum growth rate in MRS media broth with and without PAHs levels (0.01, 0.10 and 0.20µg/ml) were studied (Table 6,7 & Figure 3). Data obtained showed that, during the first 24hr (lag phase) of incubation, the presence of different concentrations (0.01, 0.10 and 0.20µg/ml) of PAHs affects the growth rate of B. bifidum. With the low level of PAHs, slightly affect was observed, which relative reduction of growth rate was 4.76%. However, when the concentration increased to 0.10µg/ml, this reduction increased to 26.19%.With the concentration of 0.20µg/ml the relative reduction was 38.10%. During the incubation period for 48 and 72hr, presence of PAHs levels delayed growth rate of B. bifidum, especially with high concentration. After the incubation period for 48hr, the relative reduction was 11.54, 29.81 and 50.96%, and after 72hr, the reduction was 9.26, 17.59 and 32.41% with PAHs at levels 0.01, 0.10 and 0.20µg/ml, respectively. On the other hand, after 96hr of incubation, the growth rate increased compared to control. The relative increases were 3.85, 11.54 and 8.87% with the levels of PAHs, 0.01, 0.10 and 0.20µg/ml, respectively.
PAHs concentrations (µg/ml) |
|||||||||
Incubation period (hr) |
Control** |
0.01 |
0.1 |
0.2 |
LSD at 5% |
||||
Count |
Log*±SD |
Count |
Log*±SD |
Count |
log*±SD |
Count |
Log*±SD |
||
24 |
42x107 |
11.36a±1.10 |
40x107 |
11.21b±1.10 |
31x107 |
10.44c±1.10 |
26x107 |
9.90d±1.03 |
1.64 |
48 |
104x108 |
16.14a±0.01 |
92x108 |
15.71b±0.01 |
73x108 |
14.91c±1.10 |
51x108 |
13.66d±1.10 |
1.8 |
72 |
108x108 |
16.27a±1.24 |
98x108 |
15.93b±1.23 |
89x108 |
15.6c±1.23 |
73x108 |
14.91d±1.10 |
1.8 |
96 |
78x108 |
15.14d±1.10 |
81x108 |
15.27c±1.24 |
87x108 |
15.52a±0.01 |
85x108 |
15.44b±0.01 |
1.8 |
120 |
77x108 |
15.09b±1.10 |
74x108 |
14.95d±1.10 |
75x108 |
15.00c±1.10 |
80x108 |
15.22a±0.01 |
2.33 |
Table 6: Growth counts of B. bifidium in MRS media broth containing different levels of PAHs during incubation at 37 ˚C for 120hr.
*Log count bacteria ± Standard Deviation (SD).
**Average of data obtained from MRS media broth containing 5µl dichloromethane/1ml MRS medium broth.
- Log10 initial count (zero time)=9.41.
-All values are means of triplicate determination.
-Means of log-count column with different letters are significantly different (p<0.05).
PAHs concentrations (µg/ml) |
|||
Incubation period (hr) |
0.01 |
0.1 |
0.2 |
24 |
4.76 |
26.19 |
38.1 |
48 |
11.54 |
29.81 |
50.96 |
72 |
9.26 |
17.59 |
32.41 |
96 |
3.85* |
11.54* |
8.97* |
120 |
3.9 |
2.6 |
3.90* |
Table 7: Relative reduction or relative increase of B. bifidium counts in MRS media broth as affected by presence of different levels of PAH.
*Relative increase.
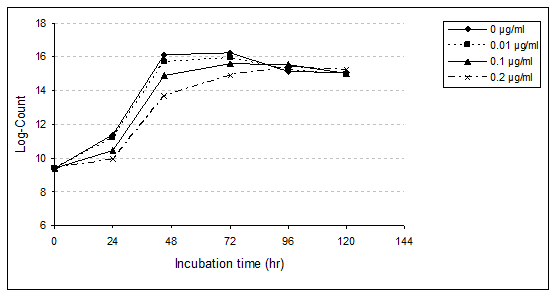
The results obtained showed that B. bifidum in MRS medium broth in untreated sample achieved to highest viable count and reached the stationary phase after the incubation period for 72hr at 37 ˚C.The same phase was attained after the same time in the presence of 0.01 and 0.10µg/ml, however its attained after 96hr with treated sample by 0.20µg/ml.
Growth rate of B. bifidum obviously inhibited in the presence of PAHs at 0.20μg/ml especially at 24, 48 and 72hr, which recorded a relative reduction, 38.10, 50.96 and 32.41%, respectively. At the end of the incubation period (120hr), an obvious decrease was recorded with treated samples of 0.01µg/ml (3.90% reduction) and 0.10µg/ml (2.60%). However, the growth rate was increased with PAHs at level 0.20µg/ml (3.9%).
Such fluctuation in growth rates of B. bifidum due to the presence of PAHs may be due to the ability of this strain to assimilate these chemicals and/or its ability to be adapted to grow under studied conditions. On the other hand, the stimulation in the viable counts of the strain through the incubation period, in PAHs treated media could be attributed to some extent to the modification in the metabolic system and/or the enzymatic multiplication system of B. bifidum cells.
Statistical analysis proved that there was a highly significant difference (p<0.01) between the tested levels on the viable count of strain grown in MRS media broth at 37 °C. Moreover, differences between the incubation periods were highly significant (p< 0.01).
Data obtained indicated that the PAHs proved to have different effects on growth rate of S. thermophilus, L. bulgaricus and B. bifidum depending upon the concentrations applied and pre-exposure time. These results are obviously related to the physiological nature of each strain. Bioavailability and other interactive effects of these PAHs on microorganisms are important to know because of these factors ultimately will determine the activity and diversity of microorganisms. After the first 24hr of incubation, the microorganisms adopted and flourished as indicated by the increased microbial activity. On the other hand, the higher concentration of PAHs in milk may cause inhibition of the beneficial microorganism activity. In the previous study by Abou-Arab et al. [8,9], the mean concentration of PAHs was 1.011µg/kg in raw milk samples and 0.198µg/kg in pasteurized milk. Data reveals that PAHs residues in milk or food items could affect the growth and activity of starter cultures used in the dairy industries as well as the fermented foods. This would then change some of the sensory properties and the quality of the resultant products. Moreover, the presence of such harmful substances in dairy products or fermented foods could be hazardous to the consumers. Therefore, safeguarding of the food industry from PAHs contamination is essential.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.