Journal of
eISSN: 2373-437X


Pokkah boeng of sugarcane caused by Fusarium verticillioides is is a destructive fungal disease in sugarcane growing regions. In China, the notable losses from pokkah boeng have occurred annually. The main objectives of this study were to:
Most of the isolates of the present study showed a similar grouping pattern in case of the three gene sequences, however, ITS and TEF-α more conservative than ATP-6ATPase gene in 15 isolates of F. verticillioides.
Keywords: Sugarcane; Pokkah Boeng; Disease incidence; F. verticillioides
Sugarcane is the most important sacchariferous crop worldwide, accounting for 70% of the total sugar production, and reaching to 86.2% in China. In China, sugarcane production is geographically distributed in two regions: Southwest China (Guangxi and Yunnan) and the South China (Fujian and Guangdong), representing one of the main economic resources in the Southwest region. Recently, Guangxi has been the most important region with 60-65% of the total of China sugar production,
Sugarcane diseases caused by different pathogens such as fungi, viruses and bacteria [1], may be considered as a major limiting issue affecting crop productivity [2]. Pokkah boeng of sugarcane is an economically important disease worldwide. Pokkah boeng is an airborne fungal disease caused by Fusarium that can lead to serious yield losses in susceptible varieties of sugarcane [3]. Survey during 2007-2013 showed an increasing trend of disease incidence, ranged from 1%-90%of the commercial cultivars in India [4]. The notable losses from pokkah boeng have occurred where susceptible varieties have been grown in a climate, in which hot and dry season is followed by a wet season. Especially in the south of China, is a typical subtropical climate region, the prevalence of the pokkah boeng disease is more severe, that serious threats to sugarcane plantation and huge losses in the production of sugar. The only effective way of controlling this disease is through resistance breeding [5]. However, the inheritance of resistance to pokkah boeng is not being fully understood.
Pokkah boeng of sugarcane caused by F. moniliforme (teleomorph F. verticillioides), causing leaf and shoot distortion and a ‘wilt’ disease [6] and it attack the top parts of a plant and young leaves start to become chlorosis. In Malaysia, the causal organism for pokkah boeng was known as F. moniliforme var. subglutinans, it also could combine with Colletotrichum falcatum and cause red rot symptom on sugarcane [7]; the other species that was reported pokkah boeng belong to F. sacchari found on sugarcane in Asia [3]. In South Africa, F. sacchari, F. proliferatum and F. andiyazi were identified as causal agents as a result of inoculation experiments in potted plants [8]. F. sacchari was also reported to be the causal organism of wilt in sugarcane [9]. To date, the species of Fusarium species complex that associated to sugarcane pokahh boeng in China has not been confirmed. To determine the taxonomic status of the species in fungus, both morphological and molecular phylogenetic analyses are complementary in modern fungal systematic [10]. The morphology of macroconidia, microconidia and conidiophores were regarded as the primary characteristic for defining in species of Fusarium [11]. In molecular phylogenetic and taxa study in Fusarium from an evolutionary point of view [12,13], mostly the multiple analyses were conducted utilizing sequences obtained from the intergenic spacer (IGS) region, the internal transcribed spacer regions 1 & 2 and 5.8S nrDNA (ITS) [14], the translation elongation factor α (TEF-α) [15], and the ATP-6ATPase [16], and β-tubulin genes can lead to more clear fungal identification. But so far, few studies on the pathogen biogeography and phylogenetic on Fusarium associated with sugarcane disease in China was reported.
In this study, we survey the natural disease incidence of Pokkah Boeng in 13 varieties of cultivars and 52 cross combinations of sugarcane in the field and extended the knowledge on symptomology and the natural infected frequency of sugarcane Pokkah Boeng. Classical and molecular microbiological methodology were applied to the F. verticillioides isolates. Molecular phylogenetic were carried out DNA sequence analysis of ITS regions, TEF-α, and ATP-6 ATPase gene, the aims of the this study were to analyze the genetic diversity of F. verticillioides originating from different sugarcane-growing areas on the basis of TEF-α, ATP-6and ITS sequences, and to examine whether the variation in the sequences of these genes could be correlated with their geographic origin.
Field survey of natural disease incidence of Pokkah Boeng
During 2008-2009, a survey was conducted in sugarcane seedling nurseries located in the Fuzhou (Fujian, Southern China) on sugarcane plants with typical symptoms of pokkah boeng disease. The disease incidence was recorded on 180 bushes (180 stems samples) selected randomly per sugarcane cultivar or cross combinations. Disease assessment was recorded three times during summer and autumn seasons. A disease severity rating scale used to record internal symptoms caused by pokkah boeng in sugarcane plants in which method of Tai et al. [17] was adopted for disease assessment with slight modification (Table 1).
Disease severity |
Disease symptoms |
0 |
Cane completely clean, no symptom |
1 |
Isolated points of rot in one cane leaf tissue |
2 |
Isolated points of rot in two cane leaves tissue |
3 |
Isolated points of rot greater than three cane leaves tissue |
4 |
Total rot and death of the whole plant |
Table 1: Disease severity rating scale used to record internal symptoms.
Sampling and isolation of strains
Sugarcane plants with typical Pokkah boeng symptoms were collected randomly from sugarcane fields in the major producing regions of China including Guangxi, Guangdong and Fujian provinces. Leaf tissues (5×5 mm) were cut using scissors from the margins of diseased lesions, were surface sterilized by dipping in 70% ethanol, followed by 30s in 0.1% HgCl2 solution, rinsed three times in sterile water, placed on potato dextrose agar (PDA), and then incubated in darkness at 28 °C. Then, single spore cultures were derived and maintained on the PDA and their identity was confirmed by morphological characterization using standard keys. All isolates used in this study are listed in Table 2.
Strain |
Species |
Genbank accession no. |
Host,Cultivar |
Location |
||
ITS |
TEF-α |
ATP-6 |
||||
FZ0801 |
F. verticillioides |
- |
- |
- |
Sugarcane;FN-22 |
Fuzhou |
FZ0802 |
F. verticillioides |
KJ765860 |
KJ765869 |
KJ765864 |
Sugarcane;FN-28 |
Fuzhou |
FZ0803 |
F. verticillioides |
KJ765859 |
- |
KJ765863 |
Sugarcane;FN-28 |
Fuzhou |
FZ0804 |
F. verticillioides |
- |
- |
- |
Sugarcane;FN-29 |
Fuzhou |
FZ0805 |
F. verticillioides |
- |
- |
KJ765862 |
Sugarcane;FN-30 |
Fuzhou |
FZ0806 |
F. verticillioides |
- |
- |
- |
Sugarcane;FN-30 |
Fuzhou |
FZ0807 |
F. verticillioides |
- |
- |
KJ765866 |
Sugarcane;ROC-16 |
Fuzhou |
GX0808 |
F. verticillioides |
KJ765857 |
KJ765867 |
- |
Sugarcane;GT00-122 |
Guangxi |
GX0809 |
F. verticillioides |
- |
- |
- |
Sugarcane;ROC-16 |
Guangxi |
GX0810 |
F. verticillioides |
- |
KJ765871 |
KJ765865 |
Sugarcane;ROC-22 |
Guangxi |
GX0811 |
F. verticillioides |
KJ765861 |
- |
- |
Sugarcane;FN-22 |
Guangxi |
GD0812 |
F. verticillioides |
- |
- |
- |
Sugarcane;ROC-10 |
Guangdong |
GD0813 |
F. verticillioides |
- |
KJ765870 |
- |
Sugarcane;FN-22 |
Guangdong |
GD0814 |
F. verticillioides |
- |
KJ765868 |
- |
Sugarcane;FN-28 |
Guangdong |
GD0815 |
F. verticillioides |
KJ765858 |
- |
- |
Sugarcane;FN-29 |
Guangdong |
GD0816 |
F. verticillioides |
- |
- |
- |
Sugarcane;FN-30 |
Guangdong |
Table 2: Provenance information for the F. verticillioides isolates analysed.
Morphological characterisation
Morphological characteristics were examined after 6 d growth of cultures initiated from a single germinated conidium. Characters examined included the shape and size of microconidia and macroconidia produced in sporodochia on PDA. Fungal structures were mounted on glass slides for microscopic examination. Single spore cultures were derived and maintained on PDA and their identity was confirmed by morphological characterization using standard protocols [18]. Drawings were made using AXIO drawing tube attached to a ZEISS ScopeA1 microscope and Scanning Electron Microscope JSM-5310LV.
DNA extraction
The selected single conidium-derived isolates were transferred to potato dextrose water (PDW) and grown as shaken cultures for 4 days at 28 °C. Total DNA was isolated from frozen fungal mycelia that was grown as shaken culture on PDW and collected by centrifugation. Briefly, a part of a mycelia was suspended in 300µl lyxsis buffer (100mM Tris-HCl, 10mM EDTA (pH 8), 2% Triton -100, 1% SDS, 100mM NaCl) and 300µl Phenol-chloroform-isoamyl alcohol (25:24:1)) and vortexed to release the DNA. After centrifugation for 5min at 5000rpm, the supernatant were mixed with 300µl chlorophorm-isoamyl alcohol (24:1), centrifuged again, The pellet washed with 70% ethanol, dried and re-suspended in 50µl dd-water and was kept at -20 °C as the purified DNA until use [12,19].
Analysis of DNA sequence of ITS, TEF-α and ATP-6from F. verticillioides isolates
The ITS, TEF-α andATP-6gene was amplified using fungus-conserved primer sequences, respectively (Table 3) [15,16,20]. Each amplification reaction included 25µl of Dream Taq Green PCR Master MIX (Thermo Fisher Science lnc., California), 5µl (10µM) of each primers and 14µl of dd-water in a final volume of 50 µl. Conditions for amplification for both regions were an initial denaturation step of 5 min at 94 °C, followed by 35 cycles of denaturation, annealing and elongation and a final elongation step of 10 min at 72 °C. For ATP-6 amplification, the 35 cycles consisted of 1 min at 94 °C, 1 min at 58 °C and 3 min at 72 °C; For the ITS and TEF-α amplification, the 35 cycles consisted of 1 min at 94 °C, 1 min at 56 °C and 3 min at 72 °C. All PCR Reactions were performed on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA), and the amplified products were sent to sequence by Sangon, Shanghai (Sangon Biotech Co., Ltd. Shanghai, China). For phylogenetic analysis of the F. verticillioides, reference DNA sequences of known isolates were retrieved from the GenBank in National Center for Biotechnology Information (NCBI). Multiple sequences were constructed by CLUSTALW 1.8 sequence alignment and phylogenetic analyses were performed with the MEGA 3.0 software (www.megasoftware.net). Phylogenetic trees were constructed based on the neighbor-joining (NJ) and Kimura 2-parameter method. Bootstrap resampling (1,000 replications) was used to measure the reliability of individual nodes in the phylogenetic tree [21,22].
Gene |
Primer |
Primer sequence 5'-3' |
ITS |
ITS1 |
5’-TCCGTAGGTGAACCTGCGG-3’ |
ITS4 |
5’-TCCTCCGCTTATTGATATGC-3' |
|
TEF-α |
EF-1 |
5'-AAGGAYGGNCARACYCGNGARCAYGC-3' |
EF-2 |
5'-ATGACACRACRGCRACRGTYTG-3' |
|
ATP-6 |
ATP6-1 |
5'-ATTAATTSWCCWTTAGAWCAATT-3 |
ATP6-2 |
5'-TAATTCTANWGCATCTTTAATRTA-3' |
Table 3: Oligonucleotide primers used in this study.
Disease symptoms and survey of natural disease incidence in field
The disease initially appeared on plants in late May or early June when daily temperature reached 25-30 °C and rainfall occurred. The early stages of infection were typified by chlorosis which appears on the basal areas of young leaves as they emerge from the spindle. The infected leaves become crumpled and the twisted leaves unfold normally and the leaves shortened. Leaf sheaths may also become chlorotic and develop irregular necrotic areas of reddish color. The most serious injury is when the fungus penetrated the growing points that caused the entire top of the plant dies and this is referred to as top rot.
In this study, we conducted a field disease survey of natural disease incidence of pokkah boeng disease of 13 sugarcane cultivars (Table 4) and 52 cross combinations of sugarcane (Table 5) in Fuzhou sugarcane seedling nurseries, the pokkah boeng disease incidence in most cultivars and cross combinations of sugarcane is between 0% -14%.The highest disease incidence and disease severity is cultivar FN02-3924 reach to 13.89% and cross combination YueNong73-204×CP84-1198 reach to 10%. However, data show that the disease incidence of cultivar Yunzhe99-91 and cross combination YunZhe89-351×CP84-1198 not yet found case of Pokkah Boeng infection in our survey, and the disease incidence is 0%.
Cultivar |
Disease severity |
Disease incidence |
||||
1 |
2 |
3 |
4 |
5 |
||
Yuegan 18 |
90.56% |
4.44% |
2.78% |
1.11% |
1.11% |
9.44% |
FG 98/296 |
95.00% |
5.00% |
0 |
0 |
0 |
5.00% |
Guiyin 6(B8) |
97.77% |
1.67% |
0 |
0 |
0.56% |
2.23% |
Yunzhe 99-91 |
100% |
0 |
0 |
0 |
0 |
0 |
Mintang 86-05 |
94.44% |
1.67% |
2.78% |
0 |
1.11% |
5.56% |
Yuegan 16 |
95.56% |
2.22% |
1.11% |
1.11% |
0 |
4.44% |
Mintang 95-261 |
98.33% |
0 |
1.11% |
0.56% |
0 |
1.67% |
RB76-5418 |
95.00% |
3.33% |
1.11% |
0.56% |
0 |
5.00% |
Mintang 96-6016 |
97.22% |
1.67% |
0 |
1.11% |
0 |
2.78% |
Gannan 99/591 |
93.34% |
2.22% |
0 |
2.22% |
2.22% |
6.66% |
ROC16 |
91.12% |
2.22% |
2.22% |
3.33% |
1.11% |
8.88% |
FN 99-20169 |
95.00% |
1.11% |
3.33% |
0.56% |
0 |
5.00% |
Table 4: Thenatural disease incidence of Pokkah Boeng in cultivar of sugarcane.
Cross combination |
Disease severity |
|||||
1 |
2 |
3 |
4 |
5 |
||
CP72-1210×YunRui 05-628 |
97.50% |
1.67% |
0.83% |
0 |
0 |
2.50% |
YunZhe 89-351×CP84-1198 |
100% |
0 |
0 |
0 |
0 |
0% |
MingTang 86-05×ROC22 |
97.50% |
1.67% |
0.83% |
0 |
0 |
2.50% |
CP65-357×YunRui 05-578 |
96.67% |
2.50% |
0.83% |
0 |
0 |
3.33% |
FN 95-1702×ROC26 |
97.50% |
0.83% |
1.67% |
0.83% |
0 |
2.50% |
FN 02-6427×ROC10 |
95.83% |
2.50% |
1.67% |
0 |
0 |
4.17% |
GuiTang 96-44×ROC10 |
95.00% |
4.17% |
0.83% |
0 |
0 |
5.00% |
Hocp93-746×ROC22 |
97.50% |
1.67% |
0.83% |
0 |
0 |
2.50% |
YueNong 73-204×CP84-1198 |
90.00% |
1.67% |
5% |
2.50% |
0.83% |
10.00% |
RB72-454×YunRui 05-189 |
95.00% |
4.17% |
0.83% |
0 |
0 |
5.00% |
ROC25×FN 91-4621 |
94.17% |
2.50% |
1.67% |
0.83% |
0.83% |
5.83% |
YueTang 91-976×Hocp91-555 |
94.17% |
2.50% |
2.50% |
0.83% |
0 |
5.83% |
CK |
92.50% |
6.67% |
0 |
0.83% |
0 |
7.50% |
YueTang 00-236×ROC22 |
98.33% |
1.67% |
0 |
0 |
0 |
1.67% |
YueTang 86-368×YunRui 03-808 |
92.51% |
5.00% |
0.83% |
0.83% |
0.83% |
7.49% |
GuiTang 94-119×YunZhe 89-7 |
96.67% |
1.67% |
0.83% |
0.83% |
0 |
3.33% |
Hocp93-746×FN 91-4621 |
96.67% |
0.83% |
1.67% |
0 |
0.83% |
3.33% |
YueTang 91-976×ROC10 |
95.00% |
2.50% |
0.83% |
1.67 |
0 |
5.00% |
YunRui 99-601×DeZhe 93-94 |
96.67% |
2.50% |
0.83% |
0 |
0 |
3.33% |
Hocp93-746×YunZhe 94-375 |
96.66% |
1.67% |
1.67% |
0 |
0 |
3.33% |
Hocp93-746×ZhanTang 74-141 |
97.50% |
2.50% |
0 |
0 |
0 |
2.50% |
ROC22×GuiTang 00-122 |
91.68% |
3.33% |
3.33% |
0.83% |
0.83% |
8.32% |
CP72-1210×DeZhe 93-94 |
97.50% |
2.50% |
0 |
0 |
0 |
2.50% |
CP84-1198×ROC22 |
94.18% |
3.33% |
0.83% |
0.83% |
0.83% |
5.82% |
ZhanZhe16×ROC22 |
96.67% |
2.50% |
0 |
0 |
0.83% |
3.33% |
FN 91-4710×ROC10 |
98.33% |
1.67% |
0 |
0 |
0 |
1.67% |
ROC11× GuiTang 91-116 |
95.00% |
2.50% |
1.67% |
0.83% |
0 |
5.00% |
YueTang 91-976×GuiTang 00-122 |
94.16% |
1.67% |
1.67% |
2.50% |
0 |
5.84% |
GuiTang 94-116×ROC22 |
97.50% |
1.67% |
0.83% |
0 |
0 |
2.50% |
GuiTang 94-119×CP74-383 |
99.17% |
0 |
0.83% |
0 |
0 |
0.83% |
FN 90-6652×YunRui 05-578 |
84.20% |
8.30% |
1.67% |
5.83% |
0 |
15.80% |
ZhanZhe 74-141×CP81-1254 |
98.34% |
0.83% |
0.83% |
0 |
0 |
1.67% |
Hocp93-746×YunRui 03-806 |
90.84% |
1.67% |
2.50% |
5.00% |
0 |
9.16% |
ZhanZhe 16×CP84-1198 |
92.50% |
5.00% |
1.67% |
0.83% |
0 |
7.50% |
ROC25×GuiTang 91-116 |
98.34% |
0.83% |
0.83% |
0 |
0 |
1.67% |
FN 94-0403×YunZhe 89-351 |
95.83% |
1.67% |
0 |
2.50% |
0 |
4.17% |
ROC20×YunZhe 94-375 |
93.30% |
5.00% |
1.67% |
0 |
0 |
6.70% |
YueTang 85-177×GuiTang 00-122 |
95.00% |
2.50% |
0 |
2.50% |
0 |
5.00% |
YunRUI 99-601×CP72-1210 |
91.67% |
5.83% |
2.50% |
0 |
0 |
8.13% |
DeZhe 93-88×YunRui 99-601 |
95.00% |
2.50% |
0.83% |
1.67% |
0 |
5.00% |
GuiTang 94-0403×ROC10 |
93.34% |
5.00% |
0.83% |
0 |
0.83% |
6.67% |
CP88-2143×YueTang 97-76 |
93.34% |
4.17% |
0.83% |
1.67% |
0 |
6.67% |
YueTang 91-976×CP84-1198 |
95.83% |
1.67% |
1.67% |
0.83% |
0 |
4.17% |
CP84-1198×YunZhe 94-375 |
90.84% |
2.50% |
2.50% |
3.33% |
0.83% |
9.16% |
CP84-1198×GuiTang 91-116 |
93.33% |
5.00% |
1.67% |
0 |
0 |
6.67% |
FN 91-4621×YunRui 05-808 |
96.67% |
1.67% |
0.83% |
0.83% |
0 |
3.33% |
FN 91-4710×GuiTang 89-240 |
95.84% |
2.50% |
0.83% |
0.83% |
0 |
4.16% |
ROC11×YunZhe 99-155 |
96.66% |
1.67% |
1.67% |
0 |
0 |
3.33% |
CP84-1198×GuiTang 91-116 |
94.16% |
4.17% |
1.67% |
0 |
0 |
5.84% |
ZhanZhe 89-113×FN95-1702 |
95.83% |
1.67% |
0 |
0 |
2.50% |
4.17% |
CP72-2086×YunZhe 94-375 |
95.84% |
3.33% |
0.83% |
0 |
0 |
4.18% |
CP84-1198×YunZhe 94-375 |
95% |
2.50% |
2.50% |
0 |
0 |
5.00% |
Table 5: The natural disease incidence of PokkahBoeng in cross combinations of sugarcane.
Our survey studies in the field observations on susceptibility of sugarcane cultivars and cross combination to pokkah boeng. Susceptibility to pokkah boeng among sugarcane was assessed by disease severity and disease incidence. Genetic or cultivar differences among sugarcane populations and non-additive genetic effect and common environment were responsible for most of the observed differences in pokkah boeng natural disease incidence [17]. The effective way of controlling pokkah boeng is through resistance breeding [5], breeders who use susceptible clones in crosses should be prepared to discard a relatively large fraction of the seedlings because of pokkah boeng susceptibility [23]. Hope of this work will help to study the genetic breeding control of resistance to pokkah boeng.
Isolate and morphological characterization
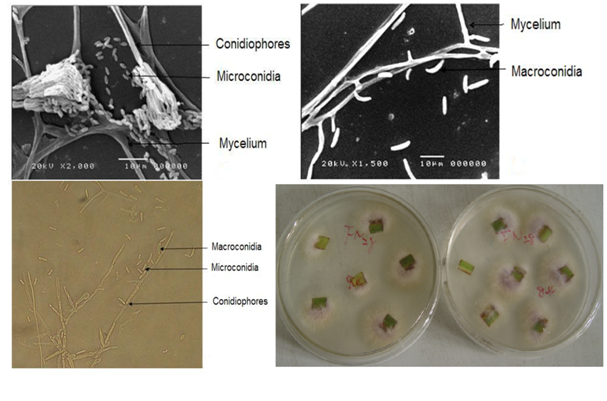
On the basis of sugarcane pokkah boeng symptom and 15 isolates of F. verticillioides were collected from different geographical areas of sugarcane in China in 2008 (Table 2). Based on the morphological criteria of F. verticillioides presented in the Fusarium Laboratory Manual [18], agreed in most respects with our observations.
Colonies on PDA was appeared cottony with radial and flat, regular growth and have shown a yellow or purple violet colony was observed, reverse with yellow to cream shades; Aerial mycelium on PDA sparse to floccose, more abundant centrally, often crateri form with bald spot in the very centre of colonies (Figure 1). Conidiophores formed in aerial mycelium or on running hyphae on the agar, erect or prostrate, at first unbranched, later branched densely terminating with ampulliformmonophialides; Microconidiaapiculate and globose, hyaline, formed abundantly in false heads; Macroconidia are inequilaterally fusoid, delicate, with an elongated, sharply curved apical cell, measure 10-20×3-5μm (Figure 1).
ITS, TEF-α and ATP-6 gene sequence analysis and molecularphylogenetics
The ITS, TEF-α and ATP-6(ATPase) gene sequences of the F. verticillioides strains were obtained and some sequences were deposited in GenBank with accession numbers from KJ765857-KJ765871 (see Table 2). A region of about 540bp (ITS), 750bp (ATP-6 ATPase), 900bp (TEF-α) was sequenced from all the isolates following PCR amplification with ITS, ATP-6 and TEF-α universal primers, respectively. Phylogenetics trees based on PCR amplification sequences from our F. verticillioides isolates and alignment with other sequences obtained from GenBank (Figure 2).

Sequence analysis of the ITS revealed that the all isolates of sugarcane Pokkah Boeng pathogen are high similar, and grouped into the a major cluster with 100% bootstrap support, ITS sequences of the isolates used in the present study shared more than 95% nucleotide sequence similarity with ITS sequences of F. verticillioides (Genbank accession No.X94166), Gibberellamoniliformis (Genbank accession No.EU1514831), F. oxysporum (Genbank accession No.FJ466709) and Gibberellafujikuroi (Genbank accession No.DQ907616) available in GenBank. However, distantly related to Ustilagoscitamineasyd (Genbank accession No.EF185083) that another sugarcane fungal pathogen and causing sugarcane smut disease. Phylogenetic analysis ATP-6ATPase sequences, there is an obvious difference between in isolated FZ0805, FZ0806 and other isolates, our F. verticillioides isolates closely related to Gibberellazeae (Genbank accession No. DQ364632), however, distantly related to F. oxysporum (Genbank accession No. AY874423), and illustrate it is not conservative in ATP-6 ATPase gene of sugarcane F. verticillioides isolates. Aligned sequences of TEF-α, our isolates and Gibberellazeae (Genbank accession No.XM388987) and F. fujikuroi (Genbank accession No. AJ 698906) clearly grouped the isolates into a cluster, with 100% bootstrap support. But there is a slight difference in many variation sites, and representing phylogenetically highly diversified of Fusarium isolates (Figure 2).
In this study, through the sequence data analysis of the ITS, TEF-α and ATP-6 ATPase region was employed, both the traditional morphological classification along with the phylogenetic analysis allowed reliable discrimination the F. verticillioides associated with pokkah boeng disease on sugarcane in China. ITS regions of 15 sugarcane F. verticillioides isolates shared 99-100% sequence identity in this study, and clustered together in phylogenetic analyses performed on a dataset comprising other sequences from anamorphic and teleomorphic taxa retrieved in BLAST searches. Each isolate representing as place of origin indicating low levels of diversity among the isolates in respect of ITS sequences. The sequence of ITS with the highest consistency, sequence of TEF-α secondly, and ATPase sequence with the lowest consistency. Specific ITS-based PCR primers for F. verticillioides can be designed based on high consistency nucleotide polymorphisms present in the ITS region, being the further step towards a better understanding and management of the sugarcane disease. The ability of the ITS region to differentiate Fusarium species and to provide accurate and rapid detection of fungi has been reported in several studies [24,25]. In the study by Maria et al. [26], multiple alignment of TEF-α gene sequence of different Fusariumspp, successfully used to detect F. fujikuroi and F. proliferatum from diseased rice tissues and seeds.
Sugarcane is one of the most noteworthy crops in China. Sugarcane pokkah boeng has become a serious threat to sugarcane production in China. The occurrence and severity of pokkah boeng have been reported from major sugarcane growing areas during all seasons, rather than only during the wet and hot summer seasons. To date, the species of Fusarium species complex that cause sugarcane pokahh boeng in China and the inheritance of resistance to pokkah boeng is not being fully understood.
The present study extends the knowledge on field disease survey of natural disease incidence of Pokkah boeng in cultivars and cross combinations of sugarcane. Knowing the suitable climatic conditions for Fusarium species associated with pokkah boeng disease on sugarcane in China, achieving a better understanding of the morphological characterization of F. verticillioides and the key factors of their shape and size of microconidia and macroconidia. Moreover, analysis of DNA sequence of ITS, TEF-α and ATP-6 ATPase gene from F. verticillioides isolates, demonstrates the sequence of ITS and TEF-1α more conservative than the ATP-6ATPase sequence in F. verticillioides, that can lead to more clear fungal identification and rapid detection of sugarcane pathogens in the future. Although the distribution of some of the F. verticillioides has been discussed in this paper, there is still a need for further biogeography research, and a diverse host range and a more comprehensive study of each group is warranted.
Financial support was provided by Guangxi special funding for distinguished experts and Funds from State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.