Journal of
eISSN: 2376-0060


Case Report Volume 10 Issue 1
National Hospital for Respiratory diseases, Sri Lanka
Correspondence: Lakshani Wasana Hakmana Kodithuwakku, National Hospital for Respiratory Diseases Welisara, Sri Lanka
Received: January 08, 2023 | Published: February 15, 2023
Citation: Lakshani WHK, Thanuja NPSM, Sumudu P, et al. Unilateral epididymal tuberculosis with scrotal fistula: a case report. J Lung Pulm Respir Res. 2023;10(1):11-13. DOI: 10.15406/jlprr.2023.10.00293
Tuberculosis (TB) affects millions of people globally every year. Although TB is predominantly a lung infection, it can affect almost any organ system in the body. Therefore, the presentation can be highly variable. Extrapulmonary TB accounts for up to 25% of all reported TB cases, and lymphoreticular and genitourinary systems are the most frequently affected. However, male genital TB infection is a rare phenomenon. A clear majority of patients with male genital TB have a concurrent pulmonary infection. We report a male patient presenting with features suggestive of usual epididymo-orchitis who later developed a frank abscess perforation through a scrotal fistula despite repeated antibiotic courses. He was later diagnosed to have epididymal TB without lung involvement. Clinicians need to be vigilant of tuberculous infection when the accepted treatment of bacterial infections invokes poor responses, especially in areas with TB endemicity.
Keywords: tuberculosis, genitourinary tuberculosis, epididymal tuberculosis, epididymo-orchitis
Tuberculosis is an infection caused by Mycobacterium tuberculosis which predominantly affects the lungs.1 However, it is a multi-systemic disease and the presentation can be highly variable among individuals.1 The gastrointestinal, lymphoreticular, skeletal, and central nervous systems and the skin are affected fairly commonly.2 It was estimated that in 2019, 10 million people worldwide developed tuberculosis, and caused 1.2 million deaths among HIV-sero negative patients and 280,000 deaths among HIV-seropositive individuals.3 There have been coordinated efforts globally in the past few decades to eradicate tuberculosis. The World Health Organization has reported that the global incidence of the disease has reduced by 1.5% per year since 2000.4 Global mortality from tuberculosis has dropped by 22% from 2000 to 2015.3 However, the vanquishment of the disease remains a challenge due to its ever-changing presentations. Here we present the case of a patient presenting with unilateral scrotal swelling, who was managed repeatedly for bacterial epididymo-orchitis and later discovered to have epididymal tuberculous infection.
Clinical vignette
A 38-year-old male patient presented to us with a two-month history of scrotal swelling on the left side. The swelling was mildly painful but did not cause warmth or redness of the overlying skin. There was no fever, urethral discharge, or dysuria. After 1 day of symptoms, he sought medical attention and underwent an ultrasound scan of the scrotum. It showed the left epididymal head and the tail to be prominent with an increased colour flow. Both testes appeared normal. The urine full report (UFR) showed 10-12 pus cells per high power field (hpf). A diagnosis of early left epididymitis was made and he was given a course of oral ciprofloxacin.
As the symptoms were persisting despite antibiotics, he underwent investigations again two weeks later. Now the UFR showed 50-60 pus cells per hpf, and the C-reactive protein level was elevated at 52 mg/L. White blood cells (WBC) were 12,000 per µL. There was no bacterial growth on the urine culture. This time he was treated with intravenous ceftriaxone for 5 days. However, repeated testing showed the UFR to be positive for a significant number of pus cells and inflammatory markers to be elevated. The erythrocyte sedimentation rate was 27mm/1st hour. The fasting blood glucose level was 98.5 mg/dL, ruling out underlying diabetes mellitus. The Mantoux test was positive at 12mm. Antibody screening for human immunodeficiency virus was negative.
His WBC, CRP, and UFR remained positive after six weeks despite several courses of antibiotics. Then, he started to develop a low-grade fever with loss of appetite and nausea. There was no specific pattern for the occurrence of the fever. A few days later, the scrotal lump started to discharge purulent material through an opening in the skin. An ultrasound scan of the scrotum showed enlargement of the left epididymis with multiple small abscesses along it, indicated by heterogeneously hypoechoic areas along the length of the organ. (Figure 1) A large abscess was noted to have perforated into the subcutaneous tissue forming a small collection in the anterolateral region. The contralateral epididymis appeared normal. Both testes were normal in size, echotexture, and vascular flow (Figure 2).
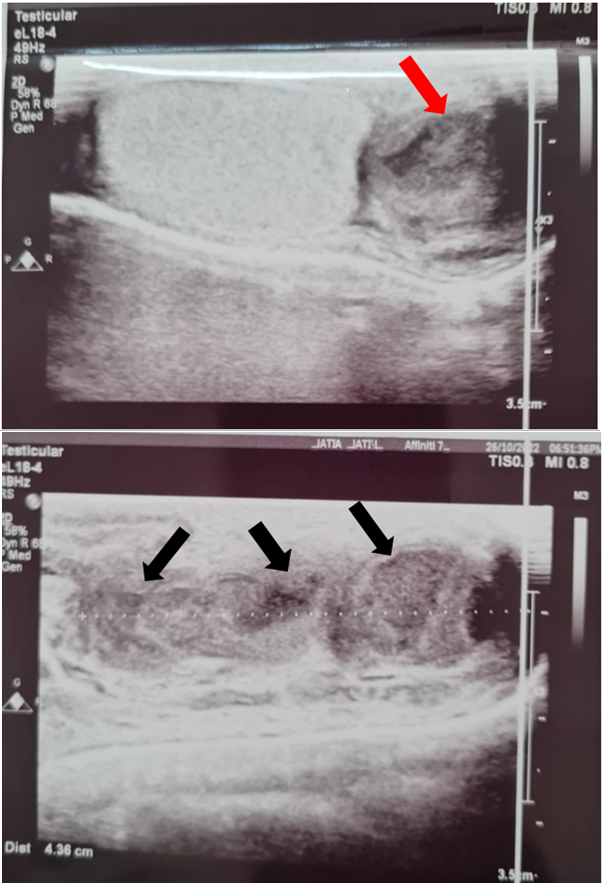
Figure 1 Ultrasound images showing abscesses in the epididymal head (red arrow) and along the body of the epididymis (black arrows).
He underwent incision and drainage of the abscesses. A transverse incision was made over the scrotum and samples were sent for bacterial and tuberculosis studies. The wound swab was negative for Gram stain and bacterial growth. The pus tested positive for AFB direct smear and real-time polymerase chain reaction (RT-PCR) for Mycobacterium tuberculosis. The tissue culture for AFB also tested positive after 4 weeks of incubation.
The patient was a non-smoker and a teetotaller. BCG vaccination had been administered in his infancy. His medical history was significant for asthma and a colonic stricture. Thickened bowel loops were noted in the pelvis while he was undergoing appendicectomy 10 years ago. A colonoscopy later showed a stricture in the mid-ascending colon, and the biopsy revealed non-specific colitis. 2 years later a contrast-enhanced computed tomography scan was done for chronic right iliac fossa (RIF) pain. It showed significant wall thickening in the terminal ileum with fat stranding. Diagnostic laparoscopy showed dilatation of the terminal ileum. A biopsy of the ileum and cytology of a RIF lymph node was negative for RT-PCR for tuberculosis. However, this presentation may be related to the current pathology and might have healed spontaneously after negative testing.
Currently, as tuberculous infection of the scrotum was confirmed, he was started on anti-tuberculosis medications (category 1 regimen). His normal chest x-ray (Figure 3), high-resolution computed tomography scanning of the chest, and negative sputum samples excluded pulmonary tuberculosis. The clinical response to medications has been satisfactory and the surgical site was still healing at the time of writing.
Mycobacterium tuberculosis is a non-spore-forming, obligate-aerobic, non-motile, intracellular bacterium.1 Its cell wall contains several lipids including cord factor, mycolic acid, and Wax-D, and this is believed to be the reason for its resistance to many antibiotics and the poor reaction with the Gram stain.1 Therefore, Gram stain cannot be utilized to identify these bacteria. The Ziehl-Neelsen stain, which uses carbol fuchsin, acid-alcohol, and another stain (methylene blue usually), is used to identify them; hence the name, “acid-fast bacilli”.1
Extrapulmonary tuberculosis (EPTB) is defined as the isolated occurrence of tuberculosis (TB) in an organ other than the lung.5 It can develop as a primary infection or a post-primary disease, where reactivation or reinfection occurs.5 EPTB accounts for 15-20% of all reported tuberculosis cases; however, the percentage rises above 50% in immunocompromised individuals.6 The clinical presentation depends on the site of involvement, and the diagnosis can be delayed as constitutional symptoms are often absent.5
Genitourinary tuberculosis is considered to be the most common form of EPTB after lymphoreticular system involvement.7 Isolated renal TB accounts for up to 80% of cases of genitourinary tuberculosis.7 However, male genital TB is considered rare, and the prostate and the epididymis are the most frequently involved organs.8 Most of the data come from case reports and case series, and it is possible that the true prevalence is underestimated as many patients are asymptomatic.9 Many patients with male genital TB have concomitant pulmonary infection (56-87%)10–12 or renal disease (49-88%)10,11,13 or both. Isolated genital TB is rare. Pulmonary TB was ruled out in our patient, but he did not undergo additional testing to check for renal involvement.
The lungs are the primary entry point for M. tuberculosis. Haematogenous spread of the bacilli then occurs with the subsequent seeding of distant organs, where highly vascular areas like the kidney and the epididymis become frequent targets.9 Primary lesions usually recover and form granulomas that can harbour dormant bacilli for decades. The majority of cases of male genital TB occur with the reactivation of a granuloma(s) after a long quiescent period. However, there have been case reports where sexual transmission has taken place and viable bacilli have been demonstrated in the ejaculate of patients with tuberculous prostatitis.14,15
The diagnosis of extrapulmonary tuberculosis can be difficult as the sensitivity of diagnostic tests remains low, and several investigations need to be combined to confirm the diagnosis.5 Epididymal TB especially cannot be differentiated from other bacterial infections based on clinical features and imaging alone.8 Patients usually present with a painless scrotal mass.9 Pain radiating to the thigh, testicle or groin may be seen uncommonly.16 The epididymis may be enlarged, non-tender and hard on examination, and advanced disease may manifest fistulae to the scrotum or the perineum.9 The classic finding of urinalysis is sterile pyuria, although some patients may grow coliform bacteria in the urine culture.17 A tuberculous epididymal abscess is difficult to be distinguished from a pyogenic abscess; however, tuberculous abscesses may be bigger and have a lower blood flow comparatively.18 This point is confirmed by our patient’s experience as he was repeatedly treated for pyogenic epididymo-orchitis before the abscesses ruptured. Tuberculosis of the epididymis sometimes may mimic a neoplasm on imaging, and there are case reports of patients undergoing orchid-epididymectomy on suspicion, and histology later revealing TB.7 Urine culture for acid-fast bacilli (AFB) remain notoriously insensitive to detect genitourinary tuberculosis, and nucleic acid amplification tests are recommended above AFB culture.9
Pharmacotherapy is the mainstay of managing all forms of male genital tuberculosis. Adjunctive corticosteroids do not appear to carry an added benefit and may lead to the reactivation of pulmonary disease.19 In the pre-chemotherapy era, genital TB was managed with transrectal ultraviolet radiation and partial or complete resection of the affected organ.20 In the present day, surgical interventions should be considered if there are complications such as non-resolving caseating abscesses and cutaneous fistulas, or if the diagnosis is not definitely established.7,9 With appropriate treatment, the mortality of genital tuberculosis is low and relapses become uncommon.9 However, cutaneous fistulae and infertility caused by the destruction of testicular tissue or the sperm outflow tract can lead to significant morbidity.15
We emphasize the importance of considering genital tuberculosis in patients presenting with scrotal masses and poorly-resolving urinary symptoms especially because Sri Lanka is an endemic country. Had our patient’s diagnosis been made earlier, morbidity arising from the scrotal fistula, surgery, and prolonged post-surgical care could have been prevented. Tuberculosis remains to be highly responsive to oral chemotherapy in most instances and prompt diagnosis and intervention could minimize considerable suffering from the patient’s point of view.
We would like to gratefully acknowledge our patient and his relatives for kindly giving us all the required details and allowing us to write about his illness. Written consent was obtained from the patient regarding publishing details pertaining to his clinical condition.
There are no conflicting interests declared by the author.

©2023 Lakshani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.