Journal of
eISSN: 2376-0060


Short Communication Volume 10 Issue 2
OncoDxRx, USA
Correspondence: Chen Yeh, PhD, OncoDxRx, LLC, 150 N Santa Anita Ave., Suite 300, Arcadia, CA 91006, USA
Received: April 01, 2023 | Published: April 12, 2023
Citation: Yeh C. Circulating cell-free transcriptomics in cancer. J Lung Pulm Respir Res. 2023;10(2):27-29. DOI: 10.15406/jlprr.2023.10.00297
Transcriptomics (or functional genomics) is a powerful tool that allow researchers to connect their knowledge of cells, biomarkers, and disease onset, hence providing novel diagnostic and therapeutic solutions and perspectives. It includes the tempo-spatial distribution, communication and interaction of key cellular mRNA biomarkers and their cross-talking networks, and their role in influencing intracellular and extracellular dynamics and signaling. Circulating cell-free transcriptomics uses data from plasma transcriptomes, or whole circulating cell-free mRNA (cfmRNA) content, to determine their roles and functions for biomarker discovery. This short communication highlights some of the technologies powering advances in the field, including current trends and innovations, and highlights future challenges and possibilities—some of which were unthinkable a few years ago.
Keywords: cell free transcriptomics, cfmRNA, plasma biomarker, cancer
One major development transforming the field of circulating cell-free transcriptomics is “inventorying and cataloging,” which involves creating an exhaustive roadmap of cfmRNA in the bloodstream. This could help establishing a detailed database of different mRNA species resided in plasma, as well as the abundance, functionality, specificity and expression patterns of these transcripts in pathophysiological states.1 The cfmRNA atlas could benefit several areas, including research and development on cancer, from early detection, monitoring to drug discovery.2–4 It could even help shed light on solid tumor minimal residual disease (MRD) and new generation cancer therapeutics via mRNA vaccine. Currently, the interplay of liquid biopsy and precision oncology has only been possible through the outputs of comprehensive tumor genome profiling. Genomic mutation (via cfDNA) and transcriptomic pattern (via cfmRNA) are orthogonal and complementary, superimposing both layers could yield much greater insights than the sum of their parts.5 Recent circulating cell-free transcriptomics-based atlasing work has been focusing on using next-generation sequencing (RNA-Seq) in connection with liquid biopsy to understand cancer pathology from the perspective of cfmRNA anatomy, combining gene expression with bioinformatics analysis.6 The gold standard approach typically involves studying tumor tissues from patients affected by cancer with significant limitations of single-site sampling at single time point, and getting the invasive access to tissues and ensuing data in these situations can present more difficulties.7 On the other hand, increasing sample pool sizes to better reflect longitudinal tumor heterogeneity and clonal evolution through liquid biopsy would be a major improvement. Furthermore, patients from different cancer types, and studying serial samples of the same patient from diagnosis onward, could help to build a more complete and specific reference atlas.
One powerful application of cfmRNA profiling is its ability to map out reciprocal interactions between tumor and immune cells as well as tumor microenvironment (TME), which is especially informative for cancer immunotherapy research.8 A heart-wrenching weakness of immune checkpoint inhibitor (ICI) drugs is our current inability to predict who will benefit from them—by the time the course of therapy is complete, there may not be enough time to choose a different therapy. Thus, predicting responses to immunotherapy, or defining good candidates for particular treatments, is an active research question—what makes “responders” different from “nonresponders"? High-resolution cell-free transcriptomic profiling is a newcomer to this search for an answer. It could be used to better track the dynamics of the immune system’s response and changes in TME by capturing cfmRNA expression signals from tumor and non-tumor tissues, instead of, or in addition to, relying mainly on the mutation or methylation status of cfDNA from tumor itself. The variety of high-dimensional cfmRNA data also provides opportunities for improving patient stratification and, as a result, the potential for improved ICI treatment.
Constant progress along different but connected tracks will put such achievements within arm’s reach. New algorithms and data analysis methods are enabling correlations of non-tumor with tumor datasets, enriching the value of entire tumor ecosystem information. There’s been an emerging recognition of the trade-off between ‘-plex’ and ‘throughput’ of cfmRNA profiling and how this pertains to patient cohort analysis. The ability to analyze large cohorts of patient samples longitudinally will likely involve discovering a panel, signature, trending or combination of biomarkers that are meaningful in upstream discovery, and then applying this to large volumes of sample testing in a high-throughput manner.
Turnaround and cost are the main focuses of cell-free transcriptomics technologies. Whole transcriptome RNA-Seq using hybrid capture technologies is a powerful research tool but can be challenged by small degraded cfmRNA input, which is common in routine clinical specimens. We have also noted a lack of dynamic range when characterizing expression of low or medium expressed transcripts, which may be required when setting clinical diagnostic thresholds or developing multivariate algorithms. The integration of a targeted cfmRNA panel with an automated high-plex workflow will enable seamless patient cohort data analysis and reporting. RT-qPCR-based platforms allow quick and seamless quantitative mapping of hundreds of cfmRNA targets—directly from plasma, without RNA-Seq. This tool generates high-quality data with high sensitivity and specificity, revealing new insights into gene expression level, pattern and functional clustering. The cutting-edge cfmRNA profiling makes it possible to look at dozens of different patient samples, at once, including multiple samples from each patient.
An interesting question is whether small laboratories will soon be able to set up large-scale cfmRNA profiling process in-house, scaling up and effectively democratizing cell-free transcriptomic techniques. The answer will depend on what will bring more value: a higher throughput of profiling, versus fewer plasma samples at a higher resolution with more biomarkers. It could also depend on the ability of technology and procedures to improve automation, while at the same time ensuring streamlined workflows. Major challenges include the need to handle, process, analyze, transfer and store very large datasets, and the ability to analyze data accurately and feasibly.
With a complete clinical picture via cfmRNA profiling technology, the process and precision of treating cancer is being redefined. Circulating cell-free transcriptomics is able to gain multilayer insights to provide the most accurate and earliest detection, tailor better treatment regimen, and thus deliver more precise and preventive medicine. It is the plasma cfmRNA innovation that enables quicker treatment decisions, better prognosis, and therapeutic approaches — laying the roadmap to personalized treatment options.
RNA transcripts serve not only as translators of genetic information, but also subjects of gene expression regulation. Compared with cfDNA mutations that are associated with tumor cells, cfmRNA biomarkers possess higher sensitivity and specificity beyond tumor itself, and have the advantage of providing dynamic and deeper insights into tempo-spatial distribution and regulatory processes including tumor clonal evolution, changes in tumor microenvironment, immune responses, and blood vessel epithelium function. All of these subtle physiological changes are prerequisite for precancerous lesion formation that can only be revealed by cell-free transcriptomics-based technology. Further, cfmRNA existed as multiple copies with various spliced variants, providing a much higher chance to be detected in blood than cfDNA. Quantitative PCR also enables traces of cfmRNA sequences to be amplified and thus captured specifically with high sensitivity. Most importantly, cfmRNA transcripts usually have stable secondary and tertiary structures, and complexed with proteins or lipids, thereby protecting them from degradation in circulation, making cfmRNA a perfect biomarker for cancer early detection, MRD, treatment selection, prognosis and mRNA therapeutics.
Forming multi-disciplinary collaborations and partnerships to manage and analyze data could be a promising way to reduce expenses and the need for resources when applying circulating cell-free transcriptomics, including sourcing the right expertise to achieve meaningful results. Such solutions could ultimately help scale up the application of functional genomics across the industry and improve the feasibility of larger-scale programs (Figure 1).
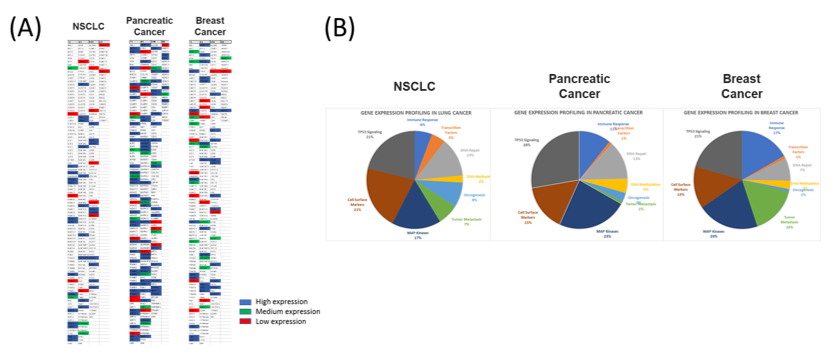
Figure 1 Plasma cfmRNA profiling by cancer type, functional cluster and expression level. (A) Gene expression heatmaps showing high-, medium- and low-expressing transcripts in different cancer types; (B) Pie charts displaying distribution of various functional classes of cfmRNA in different cancer types.
None.
The author declares that the publication was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
None.

©2023 Yeh. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.