Journal of
eISSN: 2376-0060


The preferred treatment for obstructive sleep apnea (OSA) is continuous positive airway pressure (CPAP) therapy. Although the evidence for CPAP benefits for OSA patients is compelling, acceptance and adherence remain a challenge for patients and healthcare providers.1,2 CPAP therapy is generally thought to be safe and rarely associated with complications. Breathing difficulty against a high pressure is a major complaint for some patients.2,3 For patients who cannot tolerate CPAP, Bilevel positive airway pressure (BiPAP) was thought to provide an alternative to help reduce the pressure during exhalation and perhaps improve patient comfort and compliance.4 However, there is conflicting evidence about the benefits of BiPAP over CPAP in OSA therapy.1 The increase in intrathoracic pressure during CPAP or BiPAP has the potential to produce unwanted physiological effects such as decrease in venous return, left ventricle dysfunction, increase in pulmonary vascular resistance, or decrease in lung compliance and therefore the least amount of pressure should always be used for OSA therapy. Our goal is to discuss potential changes in pharyngeal and intrathoracic pressure and in lung volume during CPAP and BiPAP treatments and how such changes may impact upper airway collapse. We will also explore the potential role of expiratory resistance devices in the treatment of OSA.
The pathophysiology of obstructive sleep apnea can be explained by collapse of upper airways secondary to the negative intra-pharyngeal pressure that develops during inspiration.4 In addition, during sleep, muscles in the mouth relax and tongue and other structures tend to fall toward the back of the mouth causing crowding in the upper airway region with more potential for upper airway obstruction. At end expiration, conditions exist that favor upper airway closure; lung volume is smallest, the pressure in the pharyngeal region is lowest, and dilator neural activity to the upper airways is least, rendering the upper airway unstable and susceptible to collapse.5 When such conditions are present, inhalation causes the pharyngeal pressure to become slightly sub atmospheric, leading to upper airways obstruction in OSA patients. The primary mechanism for improving upper airway patency during CPAP or BiPAP is related to a mechanical splinting effect due to positive pressure within the oropharyngeal space, which leads to upper airway stabilization, preventing upper airway collapse.5,6 The increase in lung volume during CPAP and dilator activity, help further to prevent upper airway collapse, but may be less important than the increase in pressure.5
Bilevel positive airway pressure (BiPAP) was suggested by Sanders et al.,4 as an alternative for OSA patients who have difficulty tolerating CPAP because they cannot get the air out of their lungs during exhalation. The rationale for using BiPAP to resolve such difficulty was that forces tending to cause upper airway collapse are less during expiration than during inspiration, hence it is acceptable to have less pressure during expiration. Gordon et al.,5 introduced the BiPAP machine that allowed independent control of inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP) and since then has been used in OSA therapy. Although the introduction of BiPAP machines provided great promise that therapy of OSA would be improved, the results were disappointing. Indeed, most studies have shown that there is little difference between BiPAP and CPAP therapy, as far as benefits, comfort, and compliance.3,7–11 BiPAP machines are 2 or 3 times more expensive than CPAP machines. Some studies found better results with BiPAP,12–14 but such findings can usually be explained by mitigating factors such as obesity, heart failure or COPD. The extra advantage of current BiPAP therapy over CPAP in OSA patients remains uncertain.1 The initial argument by Gordon and Sanders4 that forces tending to collapse upper airways during expiration are less, was subsequently shown to be incorrect, and that, on the contrary, forces tending to collapse upper airways are predominant near end expiration.15,16 Therefore one can argue that more pressure is necessary during expiration than during inspiration. In the following discussion we will discuss changes in pressure and in lung volume during various settings using a CPAP or BiPAP machines.
Figure 1 illustrates schematically the changes in pressures within the respiratory system, including changes in intra-pharyngeal pressure (dotted line), and changes in lung volume during CPAP or BiPAP. For reference, the pressure and volume changes during normal breathing are illustrated in the left panel. The numbers near the volume tracings represent the trans-pulmonary pressures (alveolar–pleural) at the beginning and end of the breath. Changes in pleural and alveolar pressures are also illustrated. The numbers near the pressure tracings are the pressures at beginning and end the breath. Regardless of the pressure settings, OSA patients always inhale spontaneously due to active contraction of the respiratory muscles, and exhale passively due to relaxation of the respiratory system. As an example, during CPAP of 10cm H2O (middle panel), pharyngeal pressure would be around 10cm H2O throughout the breathing cycle and would be associated with an increase in lung volume at end expiration. The increase in pressure and volume at end expiration are most important, because they would be responsible for preventing upper airway collapse, be it during CPAP or during BiPAP therapy. Figure 1, right panel, illustrates the changes in pressure and volume using for example BiPAP with 15cm H2O for IPAP and 5cm H2O for EPAP. At the beginning of inspiration, there is a small burst of air into the lungs when the machine switches from 5 to 15cm H2O, followed by a slow increase in volume during spontaneous inspiration. Likewise, at the beginning of expiration, there is a small burst of air out of the lungs, when the machine switches from high to low pressure, thereafter, followed by the usual slow decrease in volume during passive expiration. In this example, pharyngeal pressure and lung volume near end expiration would also be elevated, preventing potential upper airway collapse during inspiration.
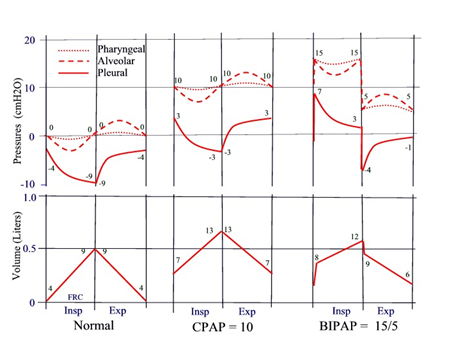
Figure 1 Schematic illustration of changes in pharyngeal pressure and lung volume during application of CPAP of 10cm H2O (middle panel) or BiPAP of 15/5cm H2O (right panel). For reference, the changes during normal breathing are also illustrated (left panel). Zero volume represents FRC during spontaneous breathing without CPAP or BiPAP. Changes in alveolar and pleural pressures are also illustrated. The numbers near the tracing represent the pressures at beginning and end of each breath. Numbers near the volume tracing represent transmural pressure (Alveolar-pleural) at beginning and end of each breath.
Figure 2 illustrates the predicted changes in pharyngeal pressures and lung volume when IPAP alone or EPAP alone is increased. The changes in pleural and alveolar pressures are also illustrated. The Figure 2 illustrates an example of the changes in pressure and volume when IPAP is set to 10cm H2O with EPAP at zero (middle panel), or vice versa (right panel). Current BiPAP machines do not allow EPAP to be set to zero, nor do they allow EPAP to be set higher than IPAP, but certainly a BiPAP machine could be designed to do that. Pressure and volume changes during a normal spontaneous breath are also illustrated for reference (left panel). With IPAP of 10cm H2O and EPAP of zero (like using an ambu bag), at the beginning of inspiration (Figure 2, middle panel), when the machine switches from 0 to 10cm H2O, a small burst of air enters the lungs, followed by slow spontaneous inhalation. At the beginning of exhalation, when the machine switches from 10cm H2O to zero, there is a burst of air out of the lungs followed by a slow passive exhalation. In this example, lung volume and pharyngeal pressure at end expiration (FRC) would not be elevated compared to normal. Therefore upper airway collapse at end expiration is not likely to be prevented. Figure 2, right panel, illustrates the changes when IPAP and EPAP are set at 0 and 10cm H2O respectively (somewhat like using expiratory resistance device such as OptiPillows™ or Provent). At beginning of inspiration, when the machine switches from 10cm H2O to zero, there is a small burst of air out of the lungs, followed by a spontaneous slow inhalation. At beginning of exhalation, when the machine switches from zero to 10cm H2O, there is a small burst of air into the lungs, followed by a slow passive exhalation. In this example, lung volume and pharyngeal pressure at end expiration would be elevated, and therefore, upper airway collapse at end expiration would be prevented as during CPAP. Despite the awkward situation, where a burst of air enters the lungs while the patient is trying to exhale, and vice versa, breathing would continue regularly. One can illustrate changes in volume and pharyngeal pressure if IPAP is elevated but remains lower than EPAP, e.g. 5 and 15cm H2O respectively. The tracings would be similar to those in Figure 2, right panel, but the volume and pressures would be shifted slightly higher.

Figure 2 Schematic illustration of changes in pharyngeal pressure and lung volume during application of BiPAP of 10/0cm H2O (middle panel) or 0/10cm H2O (right panel) using a theoretical BiPAP machine that would allow such pressure settings. Current BiPAP machines do not allow such pressure settings. The left panel, numbers and zero volume are same as in Figure 1.
The above example in Figure 2, right panel, is illustrated by using a theoretical BiPAP machine to generate positive pressure during expiration; however such an increase in EPAP can be generated spontaneously by using an expiratory resistance device, also called EPAP devices such as OptiPillows™ or Provent. Using such EPAP devices, there is compelling evidence that EPAP alone can prevent upper airway obstruction and can improve OSA, oxygenation and daytime sleepiness.16–20 The success of EPAP devices in treating OSA opened new doors and reinforced the fact that upper airway obstruction in OSA patients can be prevented by increasing the pressure during expiration. EPAP devices (such as OptiPillows™ or Provent) generate positive expiratory pressure during expiration, but the pressure during inspiration remains near atmospheric (as in Figure 2, right panel). EPAP devices have been cleared by the FDA for treatment of OSA and snoring, and have been hailed as a welcome addition to OSA therapy.21 EPAP devices are effective for OSA therapy, but also promote normal respiratory muscle activity, thus avoiding potential dependence of respiratory muscles on external pressure support. In contrast, using IPAP without EPAP (Figure 2, middle panel) has not been studied and is not clear if increasing IPAP alone is sufficient to treat OSA. IPAP and EPAP can treat OSA by different mechanisms: EPAP stabilizes the upper airways during expiration, allowing inhalation to proceed without obstruction, while IPAP can reverse upper airway closure after it has occurred. If collapse of the upper airways is prevented at end expiration with an EPAP device, most likely there would be less need for pressure support during inspiration. Nevertheless, there may be some benefit from adding a low level of IPAP to help the patient during inspiration (e.g. IPAP=5 and EPAP=10). The question is would it be useful to have a BiPAP machine that would provide such pressure settings? Such machines would treat OSA, but would also help promote respiratory muscle activity and may minimize any potential muscle dysfunction. CPAP machines usually are equipped with mechanisms that provide pressure relief during expiration (C-Flex or EPR), but perhaps a pressure relief during inspiration may also be beneficial.
We support the rationale of Sanders et al.,4 about the usefulness of a BiPAP machine to provide independent control of IPAP and EPAP, however we expand the rationale and further suggest that independent control of the inspiratory and expiratory pressure should not be limited to having IPAP greater than EPAP, but rather should allow IPAP to be set lower than EPAP or near zero. Such BiPAP machines can then be set to work like an EPAP device, but would allow more control of EPAP and IPAP levels. Current BiPAP machines may be useful in ICU patients, but may not always be best for OSA patients who are otherwise healthy. Current BiPAP machines tend to promote use of excessive IPAP (or pressure support) to reduce work of breathing. Use of such unnecessary pressure support during inspiration may promote respiratory muscle inactivity, and perhaps may cause muscle dysfunction.
There is overwhelming evidence that various modes of mechanical ventilation produce respiratory muscle dysfunction within hours in healthy animals, and in ICU patients.22,23 The diaphragm being the major inspiratory muscle but inter costal muscles could also be affected.24,25 Like other modes of mechanical ventilation, CPAP or BiPAP may also have an adverse effect on the respiratory muscle function, but is usually less evident. The effect of CPAP or BiPAP on respiratory muscles has rarely been investigated. It is commonly assumed that CPAP and BiPAP have no adverse effects on respiratory muscles, but without data to support such assumption. One study reported respiratory muscle dysfunction (inter costal muscles) in OSA patients, but CPAP (9cm H2O) for 6months did not cause additional dysfunction.24 This negative finding may be due to the fact that the pressure was low. Another study26 reported that 1 year of CPAP therapy depressed the hypercapnic ventilatory response, but wasn’t clear if that was due to weaker respiratory muscles or due to other factors. Anecdotally, patients on CPAP (or BiPAP) become dependent on the positive pressure during inspiration such that they feel it is unusually difficult to breathe during sleep if they do not use a CPAP machine. They tend to panic if they fall asleep without a CPAP machine, a feeling that was not present prior to starting CPAP therapy. Some patients describe the feeling as being “addicted” to CPAP. It is true, most OSA patients on CPAP or BiPAP therapy do not exhibit serious symptoms of respiratory muscle damage, but it may be misleading to conclude that no damage has occurred. OSA patients use the CPAP or BiPAP machine only part of the time and their respiratory muscles are likely to recover during the day from any dysfunction that may have occurred at night. Because of the cyclical nature of the injury; respiratory muscle dysfunction during the night followed by repair during the day (that maybe incomplete), there may be a residual permanent effect in the respiratory muscles, and should be taken into consideration during CPAP or BIPAP therapy.
OSA is attributed to frequent and recurring upper airway collapse during sleep. Near end expiration, mechanical conditions and neural influences are favorable to upper airway collapse. Consequently at beginning of inspiration a small decrease in pharyngeal pressure below atmospheric can easily lead to upper airway obstruction. CPAP or BiPAP machines prevent upper airway collapse by increasing the pharyngeal pressure and lung volume at end expiration, but they also tend to promote respiratory muscle inactivity. We suggest that OSA patients, who are otherwise healthy, may benefit more if the pressure during inspiration is kept near zero or at some value below the pressure during expiration. Such mode of mechanical ventilation treats OSA, promotes more respiratory muscle activity and is likely to avoid potential muscle dysfunction. A BiPAP machine that allows IPAP to be set lower than EPAP, would work somewhat like an expiratory resistance device but would provide much better control of the levels of EPAP and IPAP. EPAP devices (such as Provent and OptiPillows) cause an increase in pressure during expiration, reducing upper airway narrowing, and allowing inhalation to proceed without obstruction. EPAP devices are effective in treating OSA, but do not provide capability to control the pressure. EPAP devices are best for OSA patients who are otherwise healthy, but may not be sufficient in patients with other respiratory complications who require pressure support during inspiration. Long time CPAP users may find it difficult to switch to using an EPAP device,27 perhaps because their respiratory muscles may have become weaker and more dependent on having the pressure support during inspiration. A newly diagnosed OSA patient is more likely to get used to the expiratory resistance device much easier than a long time CPAP user. Like with CPAP or BiPAP, it may take a few days to get used to the expiratory resistance device.
In summary, EPAP devices generate EPAP without using a machine just like purse-lips breathing and are effective in treating OSA. For some OSA patients, who may need some inspiratory pressure support, it may be useful to have a BiPAP machine that allows IPAP to be increased slightly while remaining below EPAP. Having a BiPAP machine which allows such pressure settings may treat OSA with adequate EPAP, while providing minimal IPAP that would avoid compromising the functional integrity of the respiratory muscles.
None.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.
World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.