Journal of
eISSN: 2377-4312


Research Article Volume 8 Issue 1
School of Veterinary Medicine, Jimma University, Ethiopia
Correspondence: Tadele Tadess, School of Veterinary Medicine, Jimma University, Ethiopia, Tel 25 1912769075
Received: December 08, 2018 | Published: January 9, 2019
Citation: Tadesse T, Deneke Y, Deresa B. Seroprevalence of bovine viral diarrhea virus and its potential risk factors in dairy cattle of jimma town, southwestern Ethiopia. J Dairy Vet Anim Res. 2019;8(1):11?17. DOI: 10.15406/jdvar.2019.08.00235
Background
Bovine viral diarrhea virus (BVDV) is a highly contagious infectious agent of cattle populations across the world and causing a significant economic loss due to decreased performance, loss of milk production, reproductive disturbances and increased risk of morbidity and mortality. It is an envelope, positive-sense single-stranded (ss+) RNA-virus and belongs to the genus Pestivirus of the family Flaviviridae. The cross-sectional study was done form January, 2016 up to January, 2017 to estimate the seroprevalence of bovine viral diarrhea virus and its potential risk factors in dairy cattle of Jimma town, Southwestern Ethiopia. A total of 420 blood samples were collected from 45 dairy farms of the town. All sampled animals were identified by their sex, age, breeds, history of reproduction disorders (abortion, repeat breeding), parity status and history of farms by using questioner. The serum extracted from blood samples for the detection of BVDV antibody by using blocking ELISA. In this study, 51.7% (217/420) and 95.6% (43/45) seroprevalence of BVDV antibody was observed at individual and herd level, respectively. The higher seroprevalence of was observed in adult animals 55.1% (95% CI: 49.9-60.2%), dairy farms introduced new animals to their herds 100% (95% CI: 85.7-100%) and cows with history of repeat breeding as compared with cows with history of abortion 40.0% (95% CI: 24.6-57.7%) (P<0.05). In this study, age (OR: 2.5; P<0.05), repeat breeder cows (OR: 2.4; P<0.05) and introduction of new animals to herds (OR: 1.6; P<0.05) were identified as potential risk factors for the seroprevalence of BVDV. This high seroprevalence result implies as BVD is widely distributed among Jimma town dairy farms and affecting production and productivity of farms. Thus, older and repeat breeder animals should be tested for BVD and properly managed as they act as potential source of infection in addition to awareness creation about BVD for the dairy owners.
Keywords: bovine viral diarrhea virus (BVDV), cattle, elisa, jimma, prevalence, risk factors
Bovine viral diarrhea virus (BVDV) is a highly contagious infectious agent of cattle populations across the world and causing a significant economic losses due to decreased performance, loss of milk production, reproductive disturbances and increased risk of morbidity and mortality.1 The broad nature of the disease, its transmittance and lack of treatment has made it a globally enzootic and one of the most significant cattle diseases.2 It was described for the first time in United States of America as a new transmissible disease in cattle during 1946.3 The Office International des Epizootic (OIE) added bovine viral diarrhea to its list of reportable diseases in 2007, but the listing is as a reportable disease of cattle rather than as a reportable disease of multiple species.4 BVDV is relatively small (40-60nm), spherical, an envelope, positive-sense single-stranded (ss+) of approximately 12.5kb in size Figure 1.5 The genome has a single open reading frame (ORF) flanked by two un-translated regions (UTR): 5'-Npro, C, Erns, E1, E2, p7, NS2-3, NS4A, NS4B, NS5A and NS5B-3’. There are two different genotypes; BVDV-1 and BVDV-2 with several sub-genotypes.6 The viruses in the two genotypes show considerable antigenic difference from each other and within their species. There are also two biotypes of BVDV, designated as cytopathogenic (cp) and non-cytopathogenic (ncp) strains. This designation depends on their effect on tissue culture cells, where the cytopathogenic strains will cause vacuolization and cell death.7 Cattle is the primary host but serological evidence of Pestivirus infection has been found in over forty different species, for example sheep, pigs, goats, giraffe, kudu, nyala, oryx, water-buck, wild beest and African buffalo.8,9 Nose-to-nose contact between a susceptible animal and a persistently infected individual is regarded as the most efficient route of transmission.2 However; the virus can utilize indirect routes as well through the use of contaminated equipment or through insemination with BVDV infected semen.10 Bovine viral diarrhea viruses cause diarrhea, anorexia, pyrexia, oral erosion, decreased in milk production, abortion, congenital defects, poor growth, impaired reproductive performance, depression, fever, immune suppression and death. However; clinical presentations and severity of disease may vary with different strains of virus.11,12 The environmental factors and knowledge of herd management which enhance the risks of BVDV infection would make better the ability to control and impede the transmission, minimizing the unfavorable effects of BVDV infection on herd health and productivity.13 There are two diagnostic approaches to detect BVDV infection; direct tests (detection of the virus or viral components) and indirect tests (detection of the immune response to BVDV).14 The direct test includes virus isolation, antigen capture ELISA and polymerase chain reaction (PCR).15 Detection of virus specific antibodies by using different serological tests such as virus neutralization test and enzyme linked immunosorbent assay are an important ways for the indirect detection of the virus.10,16
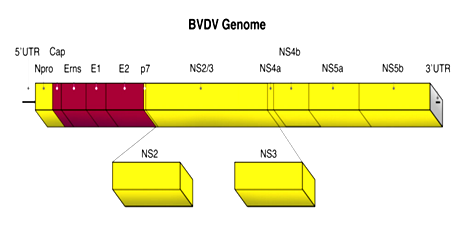
Figure 1 Genome organization of BVDV.5
Study area
The study was done in Jimma town dairy farms; southwestern part of Ethiopia from January, 2016-January, 2017. The town is located 352km Southwest of Addis Ababa between, 7º41' N latitude and 36º50' E longitudes and has an altitude of 1704 meters above sea level. The study area receives a mean annual rainfall of about 1530 millimeters that comes from the long and short rainy seasons. The mean annual minimum and maximum temperatures are 14.4°c and 26.7°c respectively with dominant warm and humid weather condition.17 Jimma town livestock populations were estimated at: 53,250 heads of cattle, 25,230 heads of sheep, 12,570 heads of goats, 10,030 heads of equine, 90,157 heads of poultry Figure 2.18
Study design
Cross-sectional study design was used in 45 randomly selected dairy farms out of 61 registered dairy farms in Jimma town from January, 2016 up to January, 2017. All of the sampled animals were Holstein-Friesian crossbreed and were housed in which food supply is by cut and carry method of feeding. Cows are hand milked with twice per day milking frequency. A very few number of farms were used natural mating where as many farms were used AI breeding systems. There was no regular vaccination and spray/dipping, but farmers took their animals for treatment whenever diseases occurred. Only dairy calves above six months of age were included for this study. Relevant individual animal data and farm level information were collected using a semi-structured questionnaire.
Sampling strategy and sample size determination
A list of registered dairy farms was collected from Jimma town livestock and fishery resource development office and Jimma town dairy cooperative enterprise office. Depending on the herd sizes, herds were classified into two categories (I-herds with ≤5 animals and II- herds with >5 animals). Animals were also grouped into two age categories; young and adult. Thereafter, one stage cluster sampling method was used due to small number of individual cows per herds. Out of 61 registered dairy farms of the town, 45 farms were selected by simple random sampling for this study. Thereafter, all animals within the randomly selected farms were included into the sample. From the previous reports of bovine viral diarrhea virus in intensive dairy farms by Asmare et al.,19 11.7% expected prevalence was used to calculate the sample size. The minimum required sample size for this study was 159 cattle by using confidence level of 95% and 5% of precision.20
Where, n, sample size; z, confidence statistic; Pexp, expected prevalence; d, desired absolute precision
According to the above formula (without considering the design effect), the minimum number of animals to be sampled are 159. To account for the design effect, the calculated sample size (n) was multiplied by the design effect (D) of 2.64 which was calculated by using a formula
Where n is average number of sampled cattle per cluster (5), an intra-cluster correlation coefficient of ρ=0.41 was reported for BVD in cattle.21 Thus, 420 cattle were selected to be enrolled in this study.
Data management and analysis
Individual animal data and history of reproductive problem were collected by interviewing the farm owners or attendants by using a semi-structured questionnaire for this purpose. Individual animal level data (age, sex, breed, parity, history of reproduction problem) and farm level data (herd size, contacts with other herds, breeding methods and introduction of new animals to herds) were obtained. Data generated from questionnaire survey and laboratory investigations were recorded and coded using Microsoft® Excel for Windows 2007 and transferred to Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM SPSS, 2011). The individual level seroprevalence was calculated as the number of seropositive samples divided by the total number of samples tested; whereas the herd level prevalence was calculated by dividing the number of positive herds by total number of herds tested. Associations between an outcome (BVDV antibodies seropositive) and explanatory variables (risk factors) for all units of analysis were investigated by using logistic regression model. The strength of the association between outcome and explanatory variables was assessed using the crude and adjusted odds ratios (OR). The explanatory variables (p<0.25) were further checked for multicolliniarity using the variance inflation factor (VIF). Variance inflation factor values of greater than 10 or Tolerance less than 0.1 were considered the cut-off points for the collinearity diagnostics.22 Variables were also tested for interaction effects using cross-product terms. Multivariable logistic regression procedures were used to model the effects of potential risk factors on outcome variables (BVDV antibodies). The backward elimination procedure was used to eliminate the factors that were not significant at p<0.05 in the overall model. Factors that were significant (P<0.05) were retained in the final model and model fit was examined by post-estimation goodness-of-fit tests, namely the Hosmer-Lemeshow test.23 Finally, those variables with P<0.05 (adjusted OR, 95% CI) were considered as a significant potential risk factors for BVDV antibody seropositive results.
The individual level seroprevalence of bovine viral diarrhea virus in the 420 cattle tested was 51.7% (95% CI: 46.9-56.4%) in which 217 animals were found seropositive. The herd level prevalence of BVDV was 95.6% (95% CI: 85.2-98.8%) that of 43 farms have at least one seropositive for BVDV antibody out of 45 sampled dairy farms. There was difference in serostatus of BVD among sex, age, parity, history of reproduction problems, herd size, introduction of new animals and breeding methods categories. Prevalence of BVD was relatively higher in female animals 53.2% (95% CI: 48.4-58.1%), adults age 55.1% (95% CI: 49.9-60.2%), >2 parity 60.4% (95% CI: 53.7-66.6%), cows without history of reproduction problems (95% CI: 56.8-67.9%), farms with more than five animals 100% (95% CI: 85.1-100%), farms introduced new animals to herds 100% (95% CI: 85.7-100%) and farms used AI 97.5% (95% CI: 87.1-99.6%) categories. In cows with history of repeat breeding, relatively higher prevalence observed compared to cows with history of abortion 40% (95% CI: 24.6-57.7%) (Table 1). There was statistically significant variation (P<0.05) in seroprevalence of BVD in age categories. Adult animals were two times (OR: 2.44; P=0.01) more likely to be infected with BVDV than young animals. Similarly, statistically significant difference in seroprevalence of BVD (P<0.05) was also observed among history of reproduction problems categories. Cows with history of repeat breeding age were almost three times (OR: 2.6; P=0.02) more likely to be exposed to BVDV than cows with no history of reproduction problems. There was also difference in seroprevalence (P=0.002) of BVD in cows with history of abortion compared to cows with no history of reproduction problems. In addition, there was association of BVD seroprevalence with dairy farms introduced animals to their herds (P<0.05). Dairy farms introduced new animals to their herds were almost two times (OR: 1.6; P=0.04) more likely to be exposed to BVDV than dairy farms those did not new animals to their herds. However, sex, herd size, parity and breeding methods were not statistically associated with BVDV seroprevalence (P>0.05) (Table 2). Variables with a P-value (<0.25) in the univariable logistic regression analysis with no multicollinearity were entered into the final multivariable logistic regression model. There was no significant interaction between variables. A Hosmer-Lemeshow goodness-of-fit value (P=0.56), indicated that the model was fit the data. The final multivariable logistic regression model of backward elimination method retained age, introduction of new animals and history of reproduction problems which were independently associated with (P<0.05) BVDV seroprevalence of Jimma town dairy cattle (Table 3).
Variables |
Categories |
Total examined |
Positive animals |
Prevalence (95% CI) |
Sex |
Female |
402 |
214 |
53.2 (48.4 58.1) |
Male |
18 |
3 |
16.7 (5.8-39.2) |
|
Age |
Adult |
361 |
199 |
55.1 (49.9-60.2) |
Young |
59 |
18 |
30.5 (20.3-43.2) |
|
Parity |
>2 Parity |
217 |
131 |
60.4(53.7-66.6) |
2 Parity |
141 |
65 |
46.1(38.1-54.3) |
|
No Parity |
44 |
18 |
40.9(27.7-55.6) |
|
History of Reproduction Problems |
Abortion |
37 |
2 |
5.4 (1.5-17.7) |
Repeat Breeding |
30 |
12 |
40.0 (24.6-57.7) |
|
No history of Reproduction Problems |
291 |
182 |
62.5(56.8-67.9) |
|
Herd Size |
>5 Animals |
22 |
22 |
100(85.1-100) |
≤5 Animals |
23 |
21 |
91.3(73.2-97.6) |
|
Introduction of New Animals |
Yes |
23 |
23 |
100 (85.7-100) |
No |
22 |
20 |
90.9 (72.2-97.5) |
|
Breeding Methods |
Bull Service |
5 |
4 |
80(37.6-96.4) |
AI |
40 |
39 |
97.5(87.1-99.6) |
Table 1 Seroprevalence of BVDV antibodies in Jimma town dairy cattle of different categories from January, 2016-January, 2017
Variables |
Categories |
Prevalence(95% CI) |
Univariable analysis |
|
Crude OR (95% CI) |
P-value |
|||
Sex |
Female |
53.2 (48.4-58.1) |
3.1 (0.76-12.6) |
0.11 |
Male |
16.7 (5.8-39.2) |
* |
* |
|
Age |
Adult |
55.1 (49.9-60.2) |
2.44 (1.2-4.8) |
0.01 |
Young |
30.5 (20.3-43.2) |
* |
* |
|
Parity |
>2 Parity |
60.4 (53.7-66.6) |
1.07(0.7-1.7) |
0.77 |
2 Parity |
46.1(38.1-54.3) |
0.97(0.02-0.04) |
0.97 |
|
No Parity |
40.9 (27.7-55.6) |
* |
* |
|
History of Reproduction Problem |
Repeat Breeding |
5.4 (1.5-17.7) |
2.6 (1.2-5.7) |
0.02 |
Abortion |
40.0 (24.6-57.7) |
0.08 (0.02-0.41) |
0.002 |
|
No Reproduction Problem |
62.5(56.8-67.9) |
* |
* |
|
Herd Size |
˃5 Animals |
100(85.1-100) |
0.78 (0.46-1.3) |
0.34 |
≤5 Animals |
91.3(73.2-97.6) |
* |
* |
|
Introduction of New Animals |
Yes |
100 (85.7-100) |
1.6 (1.02-2.4) |
0.04 |
No |
90.9 (72.2-97.5) |
* |
* |
|
Breeding Methods |
Bull Service |
80(37.6-96.4) |
0.41 (0.15-1.14) |
0.09 |
AI |
97.5(87.1-99.6) |
* |
* |
|
Table 2 Univariable logistic regression analysis of risk factors of BVDV antibodies seropositive in Jimma town dairy herds from January, 2016-January, 2017
Variables |
Categories |
Multivariable analysis |
|
Adj. OR (95% CI) |
P-value |
||
Age |
Adult |
2.5 (1.3-4.8) |
0.01 |
Young |
* |
* |
|
Reproduction Problem |
Repeat Breeding |
2.4 (1.12-5.3) |
0.024 |
Abortion |
0.08 (0.02-0.4) |
0.002 |
|
No Reproduction Problem |
* |
* |
|
Introduction of New Animals |
Yes |
1.6 (1.0-2.5) |
0.04 |
No |
* |
* |
|
Table 3 Multivariable logistic regression analysis of potential risk factors of BVDV antibodies in Jimma town dairy farms from January, 2016-January, 2017
The estimated individual level seroprevalence (51.7%) was the highest result yet reported in Ethiopian cattle. For example, 9.59% seroprevalence was previously reported by Nigussie et al.,24 in Jimma zone by using indirect ELISA, 11.7% prevalence reported by Asmare et al.,19 in central and southern parts of Ethiopia, 32.9% prevalence recently reported by Asmare et al.,25 in Ethiopian dairy cattle with history of reproductive disorders by using competitive ELISA and 32.6% prevalence by Aragaw et al.,26 in three milk sheds sample using competitive ELISA. This result was also higher than previous reports from other East African countries; 19.8% in Kenya27 and 10.7% in Sudan.28 However; the high prevalence of BVD in this study be attributed to the fact that previously infected animals has served as source of infection for dairy animal’s distribution around Jimma. In addition, it could also be related to the variation in management, study design, sample size, susceptibility and diagnostic tests performance used in the different study. Many studies were also reported almost the same result in different countries. Among these, 51.1% prevalence reported in Brazil,29 51.75%,30 both 51.58%31 and 52%32 in Iran and 51.1% in Bangladesh.33 Even though the result of this study was the highest seroprevalence yet reported in Ethiopia, it was smaller than 78.8% prevalence reported in Mexico,34 77.9% in Iran,35 66.4% in Nigeria36 and 61.61% in Croatia.37 The antibodies detected in these countries might be due to vaccination as opposed to situation in Ethiopia where there is no vaccination. This indicated as the disease was neglected and remained as one of the economically important diseases highly affecting the health and production of cattle. The variation of seroprevalence in different countries or regions could be attributable to the differences in management system (grazing practice, herd size, livestock trade, contact with other ruminants, biosecurity), types of tests used, sample size, study design and environmental condition. Many studies conducted in different countries reported that a herd is more likely to have persistently infected cattle if they are simultaneously farming with small ruminants7,38 or contact with wild animals.39,40 Residing in an area where cattle density is high is likely to lead to increased antibody prevalence.13 Many studies indicated that prevalence was higher in large herds than in small herds.3,41 Contact between animals on pasture or over fences between neighboring farms is a risk factor for BVDV infection. Purchasing breeding cattle contributes to increase number of seropositive animals in herds.39,42 In this study, the higher proportions of adult animals were seropositive compared to younger animals 55.1% (95% CI: 49.9-60.2%; P<0.05). This result was in line with other studies that reported higher prevalence of BVDV antibody in adult age than young age categories.27,43 An increase of seroprevalence as age increases possibly due to an increase in an animal’s risk of has been exposed to BVDV.44 The lower seroprevalence in young could also be due to some of the young animals investigated might be PI animals which are known to be immunotolerant to the virus and do not produce antibody against the virus to be detected by the ELISA test.43,45 It should also be noted that relatively higher numbers of adult animals were included in this study than younger animals. In this study, the higher seroprevalence was observed in cows with history of repeat breeding (possibly as a sequel to early embryonic death) compared to the cows with history of abortion (P<0.05). This result concurs with previously reported of higher prevalence of BVDV in animals with history of repeat breeding (88.9%) than animals with abortion (84.2%) history in Kenya.46 BVD infection of naive pregnant cows and heifers has been reported to lead to reproductive disorders such as early embryonic death, fetal death and mummification, birth of calves with congenital defects, calves with poor growth rates, increased age at first calving and depressed ovarian function in affected herds.47,48 Bovine viral diarrhea virus has also been reported to be fetopathogenic in cattle, thus leading to early embryonic death, repeat breeder syndrome and abortion in cattle.49 In dairy farms introduced new animals to their herds, a higher prevalence of BVD was found as compared with dairy farms not introduced new animals to their herds (P<0.05). This result concurs with previously reported purchasing breeding cattle significantly contributes to increased seropositivity compared to purchasing store cattle.42 Increasing the number of cattle purchased as well as the number of source farms will significantly increase antibody prevalence.42 In this study, adult animals were two times more likely to be infected than young animals. This result agrees with previous finding reported age as the risk factor for BVD infection.24‒44 This might reflect higher possibility of getting the virus from the environment which is shed by carrier animals. Obviously, older animals are more likely to have been exposed to the virus and as animals stay seropositive lifelong. Therefore, the herd seroprevalence is likely to be higher when many older animals are included in the sample. In dairy farms with history of introduced new animals to their herds were two times more likely infected with BVDV than those with no history of new animals’ introduction to their herds before. This result was in line with many reported from different parts of the world.50,51 This could be due to the introducing of PI animals, dams carrying PI fetuses or contact between animals from infected and non-infected herds can be transmitting the virus to naive herds.52 In this study, all of the farmers introduced new animals to their herds were have no isolation room or conducted screening test for BVDV. This was implies as they might be purchase persistently infected animals which are the risk factor for the dissemination of virus within their herds. This study showed that the risk of being infected by the BVDV was higher in cows with history of repeat breeding than cows with no history of repeat breeding. Cows with history of repeat breeding were three times more likely to be infected by BVDV than cows with no history of repeat breeding. This result is in line with previous findings in Ethiopia.25,26 In Kenyan dairy cattle also the risk of having a positive BVDV titter in repeat breeder was twice higher than no-repeat breeder.46 The study conducted on 139 repeat breeding cows in Turkey reported BVDV to be the cause in 58.2% of the cows.53 If exposure and transient infection of the dam occurs prior to embryo attachment to the endometrium, infection is avoided as BVDV does not penetrate the zona pellucida. However, following attachment embryonic infection can occur and may lead to embryo loss with the dam returning to heat.54 Therefore, BVDV might be the cause of repeat breeding and further study is recommended for confirmation of the real cause. The use of competitive ELISA for BVDV antibody testing in this study generally revealed the presence of BVDV in small scale dairy farms of Jimma town with a higher prevalence. However, based on the current study it is not possible to confirm PI status and tell the genotype of BVDV that might be predominant, whether BVDV-1 or BVDV-2.55‒57 Knowing the genotype and sub-type of BVDV is very important in term of control of the infection through vaccination approaches. BVDV distribution reported globally has shown variation in genotype and sub-type. For example, the study by Laurnes58 stated that BVDV-1 is predominant in Australia with sub-type 1c being the most prevalent, while in a study by Fulton et al.,59 revealed that the most prevalent BVDV sub-type in affected beef cattle in south central of United State of America is type 1b followed by sub-type 1a and 2a.
This study was found 51.7% and 95.6% seroprevalence at an individual level and herd level respectively. The higher seroprevalence was estimated in adult age categories, cows with history of repeat breeding compared to cows with history of abortion and farms introduced new animals to their herds. Among other suspected risk factors for BVDV infection, age, introducing of new animals to herd and animals with history of reproduction problems were potential risk factors for BVD in Jimma town dairy farms. The result of this study showed that presence BVDV antibody among dairy cattle of Jimma town as well as proofed the previous reports the presence BVDV antibodies in Jimma zone. Therefore, dairy farm owners have to isolate new animals before introducing to their herds, remove repeat breeder and old animals from herds in order to minimize the risk of viral spread in their herds. Further study needs to be done to evaluate and determine the overall prevalence status, local risk factors and economic significance of BVDV in Ethiopia. Based on the current study and previous studies, it is not possible to confirm PI status as well as tell the genotype of BVDV that might be predominant, whether BVDV-1 or BVDV-2. Knowing the genotype and sub-type of BVDV is very important in term of control of the infection through vaccination approaches.
The research and the article were financed with the funds of College Agriculture and Veterinary Medicine, Jimma University.
We would like to thank Jimma University, College of Agriculture and Veterinary Medicine for funding this research.
The author declares that there are no conflicts of interest.

©2019 Tadesse, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.