Journal of
eISSN: 2377-4312


Case Series Volume 12 Issue 2
Private Practice Veterinarian, Argentina
Correspondence: Roque Raul Lagarde, Private Practice Veterinarian, Argentina
Received: November 01, 2023 | Published: December 11, 2023
Citation: Lagarde RR. Cryogenic treatment of bladder tumors in canines: Transitional cell carcinoma and fibrosarcoma. J Dairy Vet Anim Res. 2023;12(2):132-139 DOI: 10.15406/jdvar.2023.12.00338
Bladder tumors in dogs are rare, accounting for 1-2% of all cases. Most of these tumors are malignant and of epithelial origin. This study examines various surgical approaches for the complete resection of transitional cell carcinomas of the bladder, showing a recurrence rate of 60%. Surgery may be used as an emergency therapy to relieve partial or complete ureteral obstruction, but owners should be informed that it is only palliative and associated with a high likelihood of early metastasis. Cryosurgery is a local method that relies on the tissue-destructive effects of extremely low temperatures. While intrabdominal cryosurgery is common in human medicine, it is not widespread in veterinary practice. This study presents three canine oncology cases treated through conventional laparotomy and cystotomy, employing similar cryosurgical techniques. Treatments included: i) primary transitional cell carcinoma (TCC) with previous tumor freezing, cytoreduction by debulking, and base cryoablation; ii) primary TCC with cryoablation only; and iii) poorly differentiated fibrosarcoma (STBs) with tumor excision and base cryoablation. Cold diffusion was controlled through visual observation of the 0°C isotherm and simultaneous tactile palpation. Cryoablation was performed using liquid nitrogen with a portable CRY-AC 700 Brymill device, while radiofrequency ablation was carried out with an Ellman Surgitron device. Treatment was ambulatory, well-tolerated, and resulted in rapid resolution of clinical signs.
Keywords: radiofrequency, tumor cytoreduction, cryoablation, visual control, tactile palpation, tissular sliding maneuver
Although numerous hypotheses exist regarding the etiology, the primary bladder cancer in canines remains unknown, and its nonspecific clinical manifestations create a diagnostic and therapeutic dilemma.1 Initially, it is easy to mistake a tumorous process in the bladder lumen for bacterial cystitis or urolithiasis. The classic signs of hematuria, dysuria, stranguria, and polyuria, which do not respond to conventional treatments, persist, and/or recur, suggest a reconsideration of the initial diagnosis.2–4 Blood and urine clinical analyses do not provide precise data in this regard.5 Simple or contrast radiographs, and primarily ultrasound, are methods that provide information about the implantation, possible nature, and significance. Biopsy through cystoscopy with a fiberoptic endoscope is the best option for confirming the diagnosis.
In all cases, a midline retro-umbilical laparotomy was performed. The bladder was exteriorized, and a wide and longitudinal cystotomy was made in the wall distal to the tumor implantation. Using digital manipulation and from the opposite serosa, the tumor was pushed, lifted, and exteriorized along with the nearby mucosa at the base, creating a "glove-finger" effect through the cystotomy made. This maneuver was maintained throughout the surgical procedure. Subsequently, cytoreduction was performed; involving tumor electrocoagulation with radiofrequency, and the tumor bed was frozen once. Finally, the bladder was sutured and repositioned and the abdomen was closed.
Given that the first surgical step involved a midline prepubic-retro-umbilical laparotomy, and the second step involved a longitudinal cystotomy, standard surgical materials were used.
Tumor excision was conducted through electrodessication using a 4mm tungsten loop electrode B1-4 from an Ellman Surgitron F.F.P.F radio-surgical unit, with fully rectified current for cutting/coagulation at a frequency of 3.8 MHz; electro-hemostasis was performed using a 2mm-D3-4 sphere tip electrode at a similar frequency.
The anesthetic protocol included premedication with midazolam (0.28 mg/kg/iv), induction with propofol (3 mg/kg/iv), and maintenance with 1.5% isoflurane. Anesthetic rescue was achieved with dipyrone (25 mg/kg/im) and tramadol (2 mg/kg/12 h for 3 days).
Prior freezing
The initial tissue response to freezing induces intense vasoconstriction with ischemia. Upon thawing, circulation returns to the tissues with the formation of intense intravascular obstructive thrombi. This effectively seals multiple small capillaries, significantly reducing intraoperative bleeding.
Liquid nitrogen was used as the cryogenic material applied with the portable Brymill Corp. CRY-AC #B-700 unit, along with three types of application tips: curved needle "spray" tip 103-20, short "A" spray tip 102-A, and short "B" spray tip 102-B.
The cold diffusion was monitored visually by observing the spread of the cold wave, the isotherm at 0°C, and by tactile palpation.
Post-operative care
All three patients received dexamethasone and enrofloxacin at a dosage orally for five days.
Transitional cell carcinomas (TCC)
Papillary transitional cell carcinomas (TCC) are thin projections that grow like fingers from the inner surface of the bladder towards its hollow center.1,6,7 These tumors are of low incidence, complex in nature, and often have a poor prognosis.5 Approximately 85 to 90% of these neoplasms are histologically classified as malignant, and 50 to 90% of them develop metastases. At the time of diagnosis, they often obstruct the ureters or urethra and are typically inoperable.5 The severe renal failures they cause can obscure clinical signs and lead to misdiagnoses. Only a few are identified before postmortem examination. In canines, they typically present as infiltrative papillary carcinomas with high rates of cell proliferation. Like most solid tumors, they are highly aggressive, vascular, and invasive.8 TCCs early on involve the internal iliac lymph nodes and later the lungs. They are more common in females.9,10
In the surgical treatment of TCC, it is imperative that surgical procedures be performed with care. Numerous studies confirm that sloppy surgical handling increases the spread of exfoliated neoplastic cells, leading to persistences and recurrences.
This case involves a 9-year-old female mixed-breed canine, referred for intermittent mild hematuric cystitis. Clinical examination of the abdominal cavity through palpation reveals the presence of a painless, immobile mass with a semi-soft and fixed consistency within the bladder. The ultrasonographic diagnosis reveals the presence of a heterogeneous element with irregular borders and a hypoechoic center, attached to the ventral wall and near the vesical neck, measuring 1.90 cm x 1.85 cm (Figure 1).

Figure 1 Ultrasonographic diagnosis of bladder: Heterogeneous element with irregular borders measuring 1.90 cm x 1.85 cm.
The cryosurgical option was suggested, but the owner declined. Four months later, during a follow-up ultrasound examination, an increase in the mass to 2.90 cm x 2.50 cm was confirmed (Figure 2). Faced with this situation, the owner agreed to the intervention.
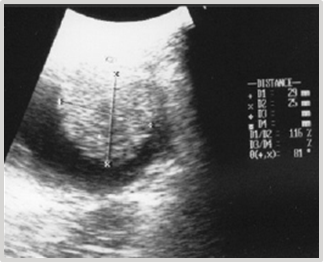
Figure 2 Ultrasonographic diagnosis of bladder four months later: Increased mass measuring 2.90 cm x 2.50 cm.
Prior freezing
After the laparotomy and cystotomy, the highly vascularized tumor mass is exposed using the "glove finger" maneuver (Figures 3–6). Given the size of the TCC (2.90 cm x 2.50 cm), the entire structure is frozen up to near the base using a short spray tip in a "zig-zag"(a) and then “concentric spiral"(b) movement. The extent of cold is monitored by tactile-visual palpation when the cold line (isotherm at 0°C) approaches to within nearly a millimeter of the mucosal base. Freezing time: 30 seconds (Figure 7) (Figure 8), thawing time: 10 minutes.
Tumor Cytoreduction: Debulking (c)
Using a cutting/coagulation current at an intensity of 3.8 MHz, two perpendicular electrodessications are performed, with the free edge of the 4mm tungsten loop electrode B1-4, from the surface to the base. These electrodessications form a cross, dividing the TCC into four equal parts (Figure 9) (Figure 10).
Using the same electrode and the same intensity, each quarter is "gently" shaved from the surface to the base. Minimal bleeding occurs. A small remnant of neoplastic tissue, approximately 1 mm in size (biopsy), is preserved and later resected along with the free edge of the compromised epithelial mucosa. Small adjacent neoplastic formations are included (Figure 11) (Figure 12).
Cryosurgery
Freezing of the tumor base begins with a short "B" spray tip, moving in a concentric "spiral" motion from the center to the periphery, with visual control of the 0°C isotherm. A 3 mm margin of adjacent healthy mucosa is included as a safety margin (d) (Figures 13) (Figure 14). The freezing of neoplastic tissue at the epithelial base includes a delicate layer of deep connective tissue.
Adjacent and also by simple proximity, the superficial, middle, and deep muscle layers are included. From the serosa, the progression of the cold front applied from the inner mucosa through the bladder wall is monitored by palpation. The distant edge of the ice sphere advances rigidly from the mucosa, including the muscle planes, but should not include the serosa (adventitia plus peritoneum). Exclusion of the serosa is achieved by performing slight, continuous finger movements of sliding back and forth in various directions on the serosa overlying the underlying subserosal connective tissue throughout the freezing.
This "tissue sliding maneuver" preserves the integrity of the serosa. The constant mobility of the serosa over the deep plane of subserosal fatty connective tissue prevents the advancement and union (cryoadhesion) between both layers through the rupture of the cryogenic structures of ice crystals in formation. The sustained movement releases the outer layer and interrupts the cryo-progression of the cold from the muscular plane, thus protecting the serosa.
Initially, a severe edema of the bladder wall complicates the exposure of the anatomical planes (Figure 16) (Figure 17). Dexamethasone is administered intravenously; followed by synthesis of the bladder, repositioning, and closure of the abdomen.
Evolution
The postoperative period progresses without particular issues, except for slight hematuria lasting 24-48 hours. The patient demonstrates a favorable course of bladder healing (Figure 18A) and early signs of hepatic metastasis at 4 months (Figure 18B).
The patient, a 9-year-old female Great Dane canine, presents with intermittent clinical signs of cystitis. Urine culture confirms its bacterial origin. The established antibiotic therapy is ineffective. The diagnosis is reconsidered. The ultrasound report indicates the presence of two closely spaced hyperechoic images, one measuring 1.61 cm x 1.16 cm and the other measuring 1.40 cm x 1.08 cm, implanted in the dorsal wall of the fundus of the urinary bladder. They involve 1/3 of its thickness (Figure 19). A laparotomy and cystotomy are performed, and the tumor is exposed through the ventral surface of the bladder using the "glove finger" maneuver.
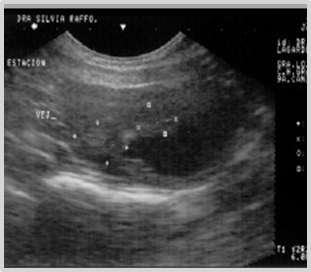
Figure 19 Ultrasonographic Image: 2 closely spaced, hyperechoic masses in the bladder measuring 1.61 cm x 1.16 cm and 1.40 cm x 1.08 cm.
Four samples are taken by biopsy, two from the tumor body and two from the nearby peripheral mucosa at the base (Figure 20).
Cryosurgery
Both formations are frozen together using a short "B" spray tip from the surface to the common base and from the center to the periphery in a "spiral" motion with tactile-visual control of the 0°C isotherm until the cold is homogenized. Approximately 1 mm of the peripheral bladder mucosa at the implantation base is included (Figure 21) (Figure 22).
With the more versatile bent "spray needle" tip 103-, an additional 2 mm of freezing is applied to the peripheral bladder mucosa (safety margin) (Figure 23). The depth of cold action is monitored by palpation using the "tissue sliding maneuver." Similarly to Clinical Case 1, after complete thawing, 5 to 10 minutes, there is intense edema of the bladder wall, which makes suturing challenging (Figure 16) (Figure 17). Dexamethasone is administered IV; the cystotomy is extended by approximately 1 cm at both ends, the bladder is closed and repositioned, and the abdomen is closed.
Evolution: The patient's progress occurs without significant clinical signs, except for mild hematuria. Symptomatic and ultrasonographic follow-up at 4, 6 months, and 1 year shows no recurrences (Figures 24) (Figure 25).
Ten months later, the animal requires a new laparotomy for septic endometritis, an opportunity that allows us to check the integrity of the bladder wall (Figure 26).
Poorly differentiated fibrosarcoma (STBs)
Malignant mesenchymal tumors like leiomyosarcoma, fibrosarcoma, myxosarcoma, and embryonic rhabdomyosarcoma are rarely present. They generally have a clustered appearance and can invade the trigone vesical area. Primary sarcomas originate in the muscle cells of the bladder wall. Soft tissue sarcomas (STBs) represent 15% of skin neoplasms, have low metastatic capacity (17%), and a moderate tendency to recur. Bladder locations are exceptional.
An 8-year-old female Dalmatian canine patient presents with clinical symptoms similar to those previously described, though as a result of urolithiasis, a common condition in this breed. Macroscopically, numerous stones of various sizes are observed. Microscopic analysis of the sediment reports abundant amorphous urate crystals. The Dalmatian breed has an autosomal recessive genetic mutation responsible for this predisposition, with a predominance in males (90%).
The medical treatment initiated for over two months fails to yield a favorable outcome. The diagnosis is reassessed. Contrast radiography and ultrasonography are requested. The reports indicate an irregularly shaped image, fixed to the ventral fundic wall, non-infiltrative, granular in appearance, with a diameter of 2.28 cm x 1.23 cm, compatible with a neoplasm (Figure 27).
The surgical times and maneuvers from the previous cases 1 and 2 are repeated, and the tumor structure is exposed through cystotomy (Figure 28). When gently pulling and lateralizing the neoplasm, the presence of a smaller diameter circular band near the base is observed (Figure 29).
With the free edge of the 4mm circular electrode in a current of cutting and coagulation at an intensity of 3.8 MHz, a total transversal electrodiathermy is performed at that level. A residual area of approximately 1mm near the base-mucosa is preserved. The central area, which has a more compact consistency, is laminated. Larger diameter vessels are clamped and electrocoagulated. Microcapillary electrohemostasis is completed with a 2mm sphere electrode D3-4, at a similar frequency (Figures 30–32).
Cryosurgery
It is frozen with a curved needle "spray" tip 103-20 from 5mm of peripheral mucosa towards the center of the tumor base, with circular movement (reverse spiral). Freezing in the central region (thinner) is done with the utmost care and accompanied by the "tissue sliding maneuver". Freezing time: 15 seconds (Figure 33), thawing time: 10 minutes.
The entire material is sent for histopathological analysis (Figure 34) (Figure 35), diagnosis: fibrosarcoma.
Evolution
The patient has shown no significant clinical signs except for mild hematuria. Follow-up ultrasonography at 3 months (Figure 36) and 12 months post-cryosurgery showed no recurrence.
After cryosurgery, all patients maintained normal urination, although there was slight hematuria during the first and second days. The treatment was outpatient, well-tolerated, and led to a rapid resolution of clinical signs.
Statistically, patients with TCC and STBs can survive without treatment for four to six months. With surgery, the period of survival can be extended, depending on the location, to between six months and one year.
According to the consulted literature, a variety of surgical and non-surgical options are proposed for the treatment of bladder neoplasms in dogs.11 Chemotherapy is the only treatment to control metastatic diseases, but it should be considered that the effects of drugs depend on the staging and performance of the patient, the cell kinetics of the tumor, and the pharmacokinetics of the drugs used. However, none have proven to be the most effective against TCC.1
In addition to recent advances in the treatment of TCC with anti-inflammatory drugs such as deracoxib and firocoxib (COX-2 inhibitors), piroxicam, and/or chemotherapy with mitoxantrone, doxorubicin, or cisplatin, used alone or in combination with surgery, it is a general opinion that the results are of limited scope.5,7,11,12 Radiation, Bacillus Calmette-Guérin (BCG), or current drugs such as vemurafenib are other important therapeutic modalities.8 Vemurafenib works by blocking the actions of the abnormal BRAF,13 inhibiting its replication, and causing cell death in localized neoplastic diseases. Vemurafenib is the latest chemotherapy applied in this pathology.11
Currently, cryosurgery is a valid alternative that can be offered to patients with serious oncological conditions, avoiding, in some cases, extensive and doubtful surgical interventions.
Ideally, the surgical intervention for any malignant bladder tumor should be planned with the perspective of resecting one, two, or more centimeters of the visible margin of the tumor's edge, which is difficult or impossible to perform in many dogs. Cryosurgery is a feasible surgical method in pathologies with critical anatomical location.14
At present, the treatment results are frustrating for colleagues and owners. The non-invasive cryogenic approach to these tumors significantly reduces the iatrogenic risk of malignant cell dispersion.
The induction of apoptosis in basal and peripheral neoplastic cells achieved through cryogenic hypothermia (-10°C) could be an alternative to explore.15 Apoptosis is an important mechanism of cell death when the temperature is not low enough to destroy the cells through direct rupture caused by intracellular ice.15 Apoptosis occurs in cells that were partially damaged at a relatively high temperature (e.g., away from the cryo-instrument) and are located on the periphery of the cryogenic lesion.15
A larger number of patients treated with this technique could confirm or refute this possibility.
None.
Author declares there is no conflict of interest in publishing the article.
None.

©2023 Lagarde. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.