Journal of
eISSN: 2377-4312


Review Article Volume 13 Issue 1
1CETAC - Veterinary Training Center, Brazil
2FAM - University Center of the Americas, Brazil
3FASIG – Faculdade de Ciências da Saúde IGESP, Brazil
4Veterinary Hospital Nosso Vet, Brazil
5IEP - Ranvier Institute, Brazil
Correspondence: André Rinaldi Fukushima, Centro Universitário da Américas, Rua Augusta, 1508, São Paulo, São Paulo, Brazil
Received: December 28, 2023 | Published: January 9, 2024
Citation: Serrano BP, Fukushima AR, Muñoz JWP, et al. Advancements in epidural anesthesia for small animals: a comprehensive literature review. J Dairy Vet Anim Res. 2024;13(1):1-10 DOI: 10.15406/jdvar.2024.13.00339
Epidural anesthesia is a loco-regional blocking technique used to prevent and control pain in pathological processes and surgical procedures, widely used in veterinary medicine. The present work aims at performing a detailed literature review, preferably of the last twenty years, on the various aspects of this technique, including anatomy of the epidural space and peculiarities in small animals, pharmacology and toxicology of the main local anesthetics used, methods for identifying the epidural space, as well as recommended doses, indications, contraindications, and complications of the procedure. With the development of this literature review, it was found that the volume, concentration, and mass of local anesthetics, in addition to knowledge of anatomy and pharmacology of local anesthetics, plays a key role in the success of the technique. Moreover, it was noticed that, with the advent of ultrasonography, the identification of the epidural space is more accurate, compared to other methods. It was concluded that epidural anesthesia is a safe procedure, but not free of possible complications and side effects, as well as any anesthetic procedure. Therefore, it is essential to evaluate each case individually, always valuing the welfare of the patient.
Keywords: regional anesthesia, neuroaxis, blockage, dogs, cats
Epidural anesthesia is a widely employed locoregional block technique in veterinary medicine, aimed at preventing and controlling pain in pathological processes and surgical procedures. Pain is known to have various deleterious effects on the body, particularly on the respiratory and cardiovascular systems, which are crucial in intraoperative and postoperative monitoring.1
In the 19th century, the development of the hypodermic needle and syringe made the application of morphine near nerves involved in painful processes feasible. At the end of this century, cocaine, the first local anesthetic, was discovered. Clinical complications associated with cocaine, such as dependency, led to the search for less toxic local anesthetics. In the early 20th century, procaine was synthesized, marking a significant advancement in regional anesthesia. Further local anesthetics, like benzocaine and tetracaine, were synthesized by 1932. The era of local anesthetics we use today, primarily lidocaine and bupivacaine from the amino-amide group, began in 1943.1,2
Drugs deposited in the epidural space primarily act on nerve roots as they exit the spinal cord and travel through the intervertebral foramina.3 Neuroaxis blockade has become a routine practice in veterinary anesthesiology, necessitating professionals to stay updated and recognize anatomical structures, physiology of the epidural space, and techniques for accurate localization, as well as the specifics, dosages, and potential side effects of the utilized drugs, ensuring patient well-being.
This work aims to conduct a detailed review of the epidural anesthesia technique through consultation of the primary literature and studies in veterinary medicine, and occasionally, human medicine, predominantly published in the last twenty years.
Anatomy of the spine of dogs and cats
Dogs and cats possess seven cervical vertebrae, thirteen thoracic vertebrae, seven lumbar vertebrae, three sacral vertebrae, and up to approximately twenty coccygeal vertebrae. The sacral vertebrae fuse into a single unit, forming the sacrum. In terms of spinal column length, the cervical, thoracic, and lumbar vertebrae constitute about 27%, 37%, and 29%, respectively, while the sacrum comprises only 7%.4
Articular processes and ligaments join the individual vertebrae. The spinal column extends from the skull to the tail end, encompassing the entire spinal cord, its meninges, nerves, blood vessels, and connective tissue within the vertebral canal, formed by the vertebral foramina of each vertebra starting from the foramen magnum in the skull and ending in the sacral canal. Spaces between vertebrae allow passage of spinal nerves, and cartilaginous intervertebral discs are situated between adjacent vertebrae.5
The supraspinous ligament attaches to the dorsal spinous processes' apices, extending from thoracic to coccygeal segments, being continuous. Interspinous ligaments connect adjacent spinous processes. The vertebral canal (Figure 1) (Figure 2) comprises the epidural space and intrathecal structures including the spinal cord, meninges, and cerebrospinal fluid (CSF). The dorsal longitudinal ligament, forming the vertebral canal's floor, attaches to vertebrae and intervertebral discs. The dorsal part of the vertebral canal consists of individual vertebral laminae and the yellow ligament (also known as flavum or inter-arcuate ligament) extending at intervertebral spaces level (Figure 3).6
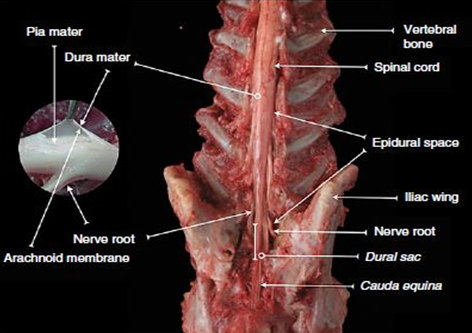
Figure 1 Dissected dog spine in lumbosacral region.
Source: Campoy and Otero.4
Caption: Dorsal view of the lumbosacral region of the vertebral canal. Note the exit of nerve roots from the spinal cord and covering by meninges.

Figure 2 Transverse section of the fifth lumbar vertebra (L5).
Source: Campoy and Otero.4
Caption: Note the epidural space (red line).
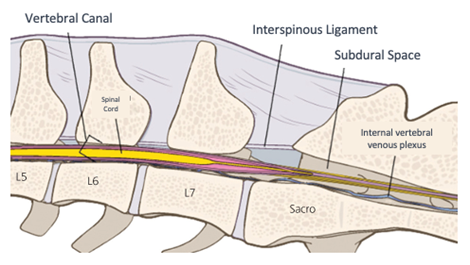
Figure 3 Drawing of the structures in the lumbosacral region.
Source: Adapted Campoy, Read and Peralta.6
Caption: Note the inter-arcuate ligament, also called yellow, an important structure for performing the epidural anesthesia technique.
The spinal cord receives blood supply from three arteries extending its entire length. The ventral spinal artery, positioned along the spinal cord's ventral surface, supplies oxygenated blood to both gray and white matter. The paired dorsolateral vertebral arteries run alongside the cord near the groove from which dorsal nerve roots emerge. The internal vertebral venous plexus, situated along the canal floor, drains blood from vertebral canal structures.6
Dorsal and ventral radicles bilaterally emerge from each cord segment and join, forming dorsal and ventral roots. Each root progresses towards the intervertebral foramen, emerging together as the corresponding spinal nerve. The dura mater accompanies these roots and nerves as they exit the vertebral canal through intervertebral foramina. Each nerve has dorsal and ventral branches. In the T1-L4 segment, two communicating branches connect the sympathetic trunk and ganglia with the spinal nerve (Figure 4).3
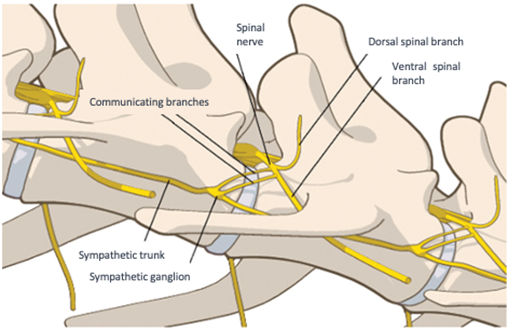
Figure 4 Sympathetic trunk.
Source: Adapted Campoy, Read, and Peralta.6
Caption: Illustration showing ganglion, sympathetic trunk, and branches of the spinal nerve (in yellow).
According to Otero and Campoy (2013),4 he spinal cord traverses the entire vertebral canal. The conus medullaris, where the spinal cord tapers, has species-specific locations. In large dogs, it extends to the sixth or seventh lumbar vertebrae, in small dogs to L6 and L7, while in cats, it reaches the first sacral segment.6 Hence, sacrococcygeal puncture is recommended for felines.5
The dural sac's distal end extends about two centimeters beyond the cord, and caudally, the cauda equina forms, corresponding to a bundle of nerve fibers from sacral and caudal segments' roots.7
As Fletcher (2013),7 describes, through its roots and nerves, the spinal cord innervates the neck, trunk, tail, limbs, and caudal and dorsal surfaces of the head. Dorsal roots transmit sensory (afferent) input, while ventral roots carry motor (efferent) output to muscles and glands (Figure 5).
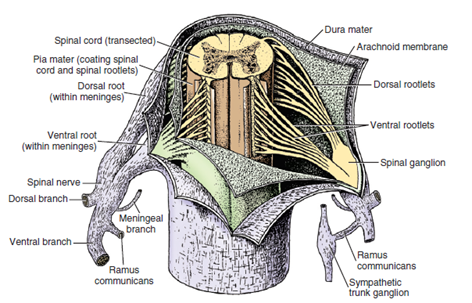
Figure 5 Spinal cord and its roots.
Source: Fletcher.7
Caption: Illustration of dissected meninges, revealing the spinal cord and the dorsal and ventral roots.
Nerve fibers' characteristics, including size and myelin presence or absence, correlate with function and conduction velocity. A group fiber are large, myelinated, and somatic, transmitting touch and pressure information (from mechanoreceptors) and modulating muscle tone and motor/reflex activity. Aδ fibers specifically convey nociception and temperature information. B group fibers, small and myelinated, are involved in autonomic function modulation, such as vascular tone control. C group fibers, small and unmyelinated, carry pain and temperature information. Aδ fibers transmit information swiftly, whereas C fibers do so more slowly.8
The lumbosacral plexus, originating from the L3-S1 segment, provides pelvic limb innervation. Complete limb blockade requires blocking this segment. T11-T13 and L1-L3 branches, via sympathetic innervation and iliohypogastric and ilioinguinal nerves, innervate the abdominal wall and peritoneum. T2-T13 spinal nerves' branches innervate the thorax. Local anesthetic progression through the epidural space and reaching respective dermatomes causes spinal root blockade (Figure 6). The sympathetic chain, originating from the T1-L4 spinal cord segment, leads to vasodilation and sometimes arterial hypotension when pre-ganglionic sympathetic blockade occurs.9
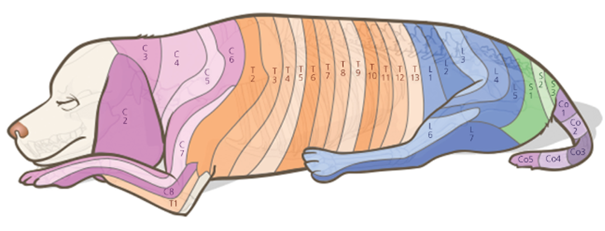
Figure 6 Dermatomes.
Source: Campoy, Read and Peralta.6
Caption: Illustration demonstrating the dermatomes present in the animal's body, corresponding to the vertebrae.
Pharmacology and toxicology of local anesthetics
Various drugs are suitable for epidural use, especially lidocaine and bupivacaine. The extent and quality of blockade depend on the volume (V), concentration (C), and mass (M) of the anesthetic (M = V x C). A larger volume, with a fixed concentration, leads to a more extensive blockade, but may not ensure adequate concentration, resulting in a low-quality blockade. Large volumes often lead to sympathetic blockade and consequent hypotension, due to pre-ganglionic sympathetic fiber blockade. In contrast, small volumes with higher concentrations produce deeper blockade. Thus, a higher concentration ensures quicker onset, greater intensity, and prolonged blockade duration, highlighting the need for an appropriate and effective dose for each procedure to minimize side effects.10
Martin-Flores,11 notes that local anesthetics (LAs) act by interrupting action potential generation and propagation in neural tissues, causing temporary loss of sensory, motor, and autonomic functions. This blockade is reversible and limited to the administration area. LAs are classified based on chemical structure, onset time, and anesthetic effect duration.
Most LAs comprise three parts: a lipophilic radical, a hydrophilic radical, and an intermediate chain (ester or amide). The lipophilic radical facilitates nerve cell membrane passage, while the intermediate chain influences drug synthesis and metabolism. Ester links lead to rapid hydrolysis and short effects, while amides have slow biotransformation and prolonged effects. The hydrophilic radical determines action speed and is influenced by the environment's pH.12
Anesthetics cross nerve membranes by diffusion following a concentration gradient, dependent on the drug's liposolubility, environmental pH, and concentration. The more liposoluble the drug, the greater its potency and potential for undesirable effects. The ionic form interacts with sodium channels, causing blockade (Figure 7). As LAs are weak bases with a pKa of 7 to 9, the ionic form predominates, hindering membrane passage. Thus, LAs overcome barriers in their non-ionized form, with the ionized form responsible for the anesthetic effect.9,11
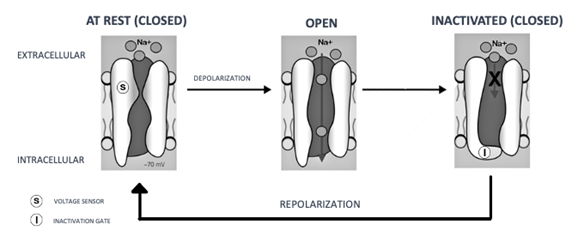
Figure 7 Mechanism of action of local anesthetics.
Source: Adapted from Grimm et al.6
Caption: Note the blockade of sodium channels.
Peak plasma LA concentrations and time to reach them are governed by systemic absorption rates, determined by the vascularization of application sites.13
At the deposition site, various structures compete for LAs, including nerve tissue, fat, blood, and lymphatic vessels. Only a small fraction remains for primary action. Once absorbed, they bind to plasma proteins and distribute to tissues. Drugs with high liposolubility and protein binding rates disappear from the bloodstream more slowly. It's vital to note that drugs with high protein binding will have an increased free fraction in patients with hypoproteinemia, contrary to those with low protein binding rates.14
Valverde15 states that LAs block nociceptive Aδ and C fibers, effectively preventing pain from surgical procedures. Sympathetic B fibers and motor Aβ and Aα fibers are also affected, leading to vasodilation, proprioception changes, and motor blockade. B fibers are blocked at low concentrations; C fibers require higher concentrations than Aδ fibers but are blocked by differential diffusion before myelinated Aδ fibers due to their unmyelinated nature; Aα fibers are more resistant. Clinically, the blockade order is B, C, Aδ, and Aα fibers.
Local anesthesia, including epidural, is generally safe, but toxicity can occur, especially with improper administration. Toxicity can be local or systemic, with the central nervous system (CNS) or the cardiovascular system (CVS) more frequently involved. CNS is more sensitive to adverse effects than CVS. Hematological (such as methemoglobinemia) or allergic reactions are also reported. The assessment of potential intoxication should be guided by clinical presentation (Table 1), with more potent drugs having a higher capacity for toxic effects.13–15
|
Clinical Sign |
Description |
|
Nystagmus |
No additional symptoms or specific observations. |
|
Muscle Tremors |
No additional symptoms or specific observations. |
|
Tonic-Clonic Seizures |
No additional symptoms or specific observations. |
|
Tremors or Seizures |
Increased levels of lactic acid and hypoxia may be observed after the onset of seizures. |
|
Generalized CNS Depression |
Drowsiness, unconsciousness, coma. |
|
Hypotension |
Depressed systolic function, vasodilation, bradycardia, other arrhythmias. |
|
ECG Changes |
QRS complex widening, inversion, bradycardia, premature ventricular complexes, ventricular tachycardia, ventricular fibrillation. |
|
Death |
No additional symptoms or specific observations. |
Table 1 Clinical signs of local anesthetic toxicity
Source: Martin-Flores.9
Martin-Flores,9 mentions that high plasma LA levels can result from high doses or unintentional intravascular administration. Toxicity can also arise if drug biotransformation and/or elimination are slower than normal, such as in hepatic or renal insufficiency.
Futema10 indicates that cardiovascular effects of these drugs, easily compensated in healthy patients, can be detrimental in those with cardiac alterations. Hence, animal evaluation before epidural anesthesia or any anesthesia-involved procedure is essential.
The Table 1 – show the clinical signs of local anesthetic toxicity and Table 2 show a guidelines for the treatment of local anesthetic toxicity.
|
Condition |
Treatment |
|
CNS Toxicity |
1. Intubate the patient, administer O2 and ventilation. 2. Treat seizures with a benzodiazepine. |
|
Cardiac Arrest |
1. Initiate cardiopulmonary resuscitation immediately 2. Administer epinephrine in low doses (≤ 1 μg/kg IV). 3. Avoid the administration of lidocaine, vasopressin, calcium channel blockers, and beta-blockers; In the case of ventricular arrhythmias, use amiodarone. 4. Administer a 20% lipid emulsion IV: - Initial bolus of 1.5 to 4 mL/kg over 1 min. - Continue with 0.25 mL/kg/min for 30 to 60 min. - If there is no response, administer another bolus of 1.5 mL/kg (up to a maximum of 7 mL/kg). - The infusion may be continued at 0.5 mL/kg/h until clinical signs improve (maximum of 24 hours). |
Lidocaine
Lidocaine, a widely used local anesthetic in veterinary medicine, is a precursor in the amino-amide class. Characterized by rapid onset and intermediate duration (1-2 hours), it is typically used in 1% and 2% concentrations. For epidural administration, lidocaine is recommended for short-duration surgeries (up to 1 hour), and for surgeries extending up to 2 hours, a combination with bupivacaine is advised. Caution is advised in pregnant patients as it can cross the placental barrier and potentially cause cardiac alterations in the fetus. Besides epidural anesthesia, lidocaine is used for antiarrhythmic purposes, particularly in treating ventricular arrhythmias in small animals. Its toxic effects may include drowsiness, hypotension, muscle tremors, nausea, and vomiting.9,10,13
Bupivacaine
Bupivacaine, known for its slower onset but significantly longer-lasting anesthetic and analgesic effects, is about 3 to 4 times more potent than lidocaine. Commonly used in veterinary medicine, it is recommended for epidural administration in long-duration surgeries (2 hours or more) at a typical concentration of 0.5%. Notably, bupivacaine exhibits higher cardiotoxicity compared to other local anesthetics, a risk further increased in patients treated with beta-blockers and digitalis. Its cardiovascular effects can include tachycardia, ventricular fibrillation, and atrioventricular block (Table 3) (Table 4).9,10,13
|
Local anesthesic |
Relative vasodilatory activity |
Liposolubility |
Maximum blood levels |
|
|
|
|
|
Dose in mg |
Concentrations in mg/m-1 |
|
Lidocaine |
1,0 |
2,9 |
300mg |
1,4 |
|
Bupivacaine |
2,5 |
27,5 |
150mg |
1,0 |
Table 3 Comparison between lidocaine and bupivacaine regarding vasodilatory activity and liposolubility in absorption in the epidural space
Source: Massone and Cortopassi.13
|
Local anesthetic and concentration (%) |
Onset of action (min) |
Approximate duration (h) |
|
Lidocaine 2% |
5 |
2-4h |
|
Bupivacaine 0.5% |
10 |
4-6h |
Table 4 Commonly used short- and long-acting local anesthetics in epidural anesthesia for dogs and cats
Source: Martin-Flores.17
Adjuvant drugs in epidural anesthesia
Adjuvant drugs such as opioids, α2-agonists, and ketamine are often combined with local anesthetics to enhance analgesia (Table 3). Opioids are particularly prevalent in veterinary epidural anesthesia.3
Opioids
Opioid selection for surgical procedures and postoperative analgesia depends on the pain level and cranial dispersion expected during surgery, with more potent opioids required for more painful surgeries.3,9,10 Valverde (2008)3 lassifies opioids by lipophilicity, ranging from highly lipophilic (e.g., fentanyl, sufentanil) to hydrophilic (e.g., morphine). The addition of opioids can reduce the required dose of local anesthetics and their motor effects. Opioids exert their analgesic effects in the epidural space either by interacting with opioid receptors in the spinal cord's dorsal horn (spinal analgesia) or in the brainstem (supraspinal analgesia), with hydrophilic agents like morphine primarily inducing spinal analgesia.9
The combination of lidocaine or bupivacaine with morphine is common, with morphine providing long-lasting analgesia (up to 24h) without motor blockade. Fentanyl, due to its lipophilic nature, short latency, and satisfactory analgesia, is preferred during surgery. Methadone, although less commonly used, has been shown to decrease isoflurane's minimum alveolar concentration for longer than when administered intravenously.16–18
α2-Adrenergic agonists
Dexmedetomidine, an α2-agonist, is increasingly used in small animal veterinary medicine, gradually replacing xylazine due to its fewer adverse effects.19 The combination of local anesthetics with α2-agonists enhances and prolongs analgesia through actions on α2 receptors in the spinal cord's dorsal horn, inhibiting Aδ and C fiber activity and reducing nociceptive stimuli proliferation.19,20 The systemic absorption of α2-agonists in the epidural space mirrors that of systemic doses, influencing cardiovascular and respiratory systems, along with sedative effects.19,21
Ketamine
Ketamine, an NMDA receptor antagonist in the spinal cord's dorsal horn, plays a role in reducing sensitization and nociception, particularly in chronic pain. It produces motor and sensory blockade when administered epidurally and has minimal hemodynamic effects in dogs. The combination of ketamine with lidocaine has shown prolonged analgesic effects in cats, though severe ataxia was observed. Despite some studies highlighting ketamine's analgesic potential, its use is cautioned against until more information on its neurotoxic effects is available20–24 emphasize that ketamine should not be administered epidurally or intrathecally until further studies report its neurotoxic effects.
Fukushima et al.25 demonstrated ketamine's safety in cardiovascular and urinary systems through a study involving catecholamine and indoleamine detection in rat cardiac muscles (Table 5).
|
|
Sympathetic Blockade (B fibers) |
Motor Blockade (Aα fibers) |
Sensory Blockade (Aδ and C fibers) |
Tactile Sensory Blockade (Aβ fibers) |
|
Local anesthetics |
+++ |
+++ |
+++ |
+++ |
|
Opioids |
⸺ |
± |
++ |
⸺ |
|
A2-agonists |
⸺ |
+ |
+++ |
++ |
|
Ketamine |
⸺ |
++ |
++ |
++ |
Table 5 Action of drugs used in the epidural space on different nerve fiber
Source: Valverde, et al.,3
Note:
Abbreviations and meanings:
⸺, no effect;
±, in toxic doses;
+, ++, +++, minimal, moderate, and maximum effect, respectively."
Considerations on the technique and methods of identification of the epidural space
Epidural anesthesia in dogs and cats can be performed under sedation or general anesthesia, either for post-surgical pain relief or intraoperative analgesia. Regardless of the timing, standard sedation and anesthesia protocols should be followed. This includes placing an intravenous catheter and monitoring vital signs like the electrocardiogram, oximetry (SpO2), and blood pressure. Additionally, preparing for airway access and oxygen provision is essential. Using short-duration intravenous agents like propofol, the depth of sedation can be safely controlled. In trauma cases, deep sedation or general anesthesia may be necessary, particularly if performed pre-surgically, where it's usually done under general anesthesia.17
Several methods exist to confirm needle placement in the epidural space, including loss of resistance, the hanging drop technique, Baraka's technique, ultrasound, electrostimulation26 and pressure wave measurement.27 While devices can enhance certainty about needle positioning, the anesthesiologist's knowledge of anatomy and training remains vital.4,28
Anatomical references and patient positioning
The lumbosacral epidural technique involves palpating the lumbosacral junction to identify the injection site, typically felt as a large space in the midline, aligned with the iliac wings (Figure 8). The patient should be placed in sternal recumbency with pelvic limbs forward (Figure 9), enlarging the distance between lumbar vertebrae and sacrum, thus enlarging the epidural space. In obese animals, lateral recumbency can facilitate palpation of the midline.18 The puncture site, located between the spinous processes of L7 and S1 (medial sacral crest), requires trichotomy and surgical antisepsis. Using sterile gloves, the anesthesiologist should insert the Tuohy needle perpendicularly to the dorsal midline skin, just caudal to the spinous process of L7, at a 45° angle (Figure 10). The needle, with external centimeter markings and a curved, blunt bevel (Figure 11), provides tactile feedback as it advances through the ligaments, interspinous ligament, and yellow ligament, accessing the epidural space.4,17,18
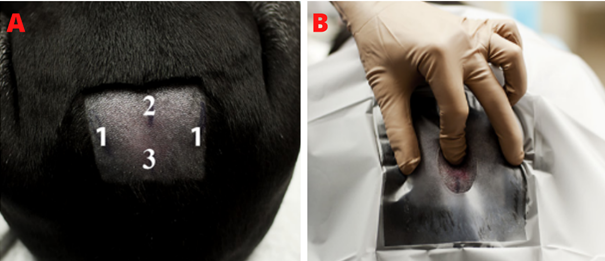
Figure 8 Anatomical references and puncture site.
Source: Garcia-Pereira et al.,28
Caption: (A) Anatomical references; 1: iliac wings, 2: spinous process of L7, 3: spinous process of S1. (B) Palpation of puncture site for lumbosacral epidural performance, the index finger points to the exact location.
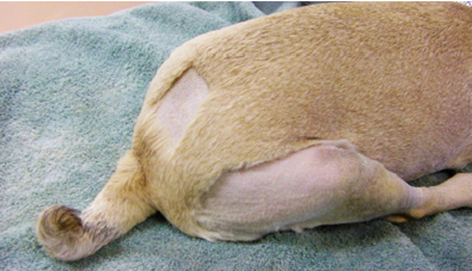
Figure 9 Patient positioning.
Source: Otero and Campoy.4
Caption: Sternal recumbency and pelvic limbs positioned forward.

Figure 10 Puncture between l7 and s1.
Source: Personal Archive.
Caption: Moment of puncture in the lumbosacral region. Note the angle of the Tuohy needle (black arrow) in relation to the red dashed line, approximately 45º.

Figure 11 Tuohy needle.
Source: Campoy, Read and Peralta.6
Caption: 20 gauge Tuohy needle Note bezel with curved and blunt tip.
Particularly in felines, due to the extension of the conus medullaris to S1 or S2, sacrococcygeal epidural is often indicated. This technique involves identifying the puncture site (Figure 12) by moving the tail up and down while palpating the sacrococcygeal area. The first movable space at the caudal end of the sacrum corresponds to the sacrococcygeal or the first intercoccygeal space, suitable for puncture as there's no risk of accidental subarachnoid puncture in this area.5,10,18
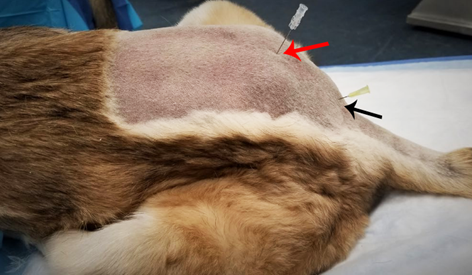
Figure 12 Lumbosacral epidural vs. Sacroccosygeal.
Source: Grubb and Lobprise.18
Legend: Comparison of feline cadavers in relation to the puncture site of the lumbosacral epidural (red arrow) and sacrococcygeal epidural (black arrow). Notice pelvic limbs positioned forward.
The needle should be inserted at an approximately 45° angle to the skin surface, cranially, and advanced slowly into the space. The needle often requires repositioning to pass through the ligament flavum, marked by a click, although its absence should not be mistaken for incorrect puncture, especially when using sharper regular hypodermic needles. Due to minimal resistance in this region, the loss of resistance technique is suitable.4,6,18
Hanging drop and loss of resistance techniques
The correct positioning of the needle in the epidural space can be confirmed using the hanging drop technique, which involves placing a few drops of sterile saline into the hub of the Tuohy needle before advancing it to the ligamentum flavum (Figure 13). Entry into the space results in the droplet being aspirated due to negative pressure, which can vary between -6 and 15 mmHg in dogs, influenced by anatomy and individual differences.27,29 The success of this technique may vary with the patient's positioning and size, with reports of false positives in certain positions. The hanging drop technique is more effective in medium to large breed dogs but less reliable in smaller dogs and cats, where negative epidural space pressure doesn't consistently result in positive aspiration.30

Figure 13 Pendant drop technique.
Source: (A) Personal archive; (B) Garcia-Pereira , et, al.,28
Legend: (A) Time of application of sterile saline; (B) Gout before being aspirated.
The loss of resistance technique involves using a syringe filled with saline or a small amount of air to check the needle's position in the epidural space (Figure 14). Resistance to the injection of air or fluid is felt as the needle advances through the ligaments. Upon passing the ligamentum flavum, a click and sudden loss of resistance are noticed. False positives may occur if the needle enters epidural fat, and false negatives can arise from needle obstruction.4
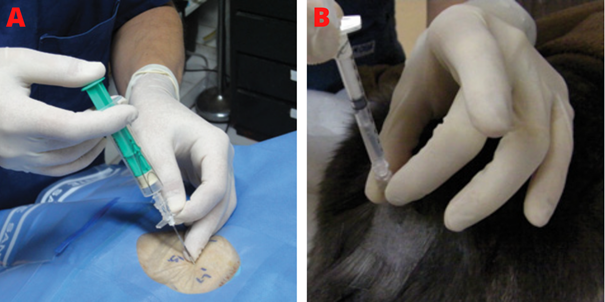
Figure 14 Resistance loss technique.
Source: Otero and Campoy.4
Caption: (A) Loss of resistance syringe to identify the epidural space of a dog. As the needle progresses slowly, slight pressure is applied to the syringe plunger; (B) Administration of local anesthetic in cat. Once the syringe is connected to the needle, an air bubble is used to assess the resistance to injection. During injection, if the air bubble is compressed, resistance is being detected and the injection must be stopped.
Baraka's technique
The Baraka technique, or "drip infusion," uses a saline bag connected to a drip set linked to a Tuohy needle for a constant fluid infusion (Figure 15). The patient should be placed in sternal recumbency, and the Tuohy needle is inserted at a 45-60° angle to the skin in the midline. The needle passes through the skin, subcutaneous tissue, supraspinatus, and interspinous ligaments. When the ligamentum flavum is punctured, resistance is felt, and the infusion stops. As the needle advances through the ligamentum flavum into the correct space, the infusion flows again, indicating the epidural space. The drip rate may vary due to individual differences in epidural pressure. No resistance during puncture and the absence of tachycardia or spinal anesthesia confirm proper epidural placement.31
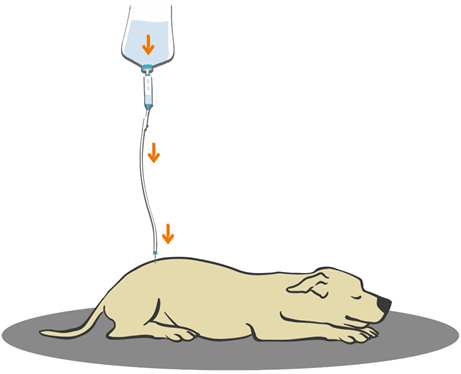
Figure 15 Illustration of baraka's technique on dog.
Source: Personal archive.
Caption: Schematization of the drip infusion technique. The orange arrows indicate the action of gravity, which causes the droplets to descend, in addition to the pressure present in the epidural space itself.
Ultrasound
Ultrasound use in veterinary regional anesthesia allows real-time needle visualization and identification of key anatomical structures like vessels, muscles, the epidural space, and the ligamentum flavum. Image quality depends on the ultrasound device, transducer, and veterinarian's proficiency.6,28 Ultrasound frequencies range from 2 to 15 MHz, with a correlation between tissue depth and image quality. Low-frequency waves penetrate deeper but with less resolution, while high-frequency waves offer better resolution but do not penetrate as deeply.32 During ultrasound, artifacts like the acoustic shadow can occur, which is relevant during guided epidural procedures.33,34
For ultrasound-guided epidural puncture, the patient is placed in sternal recumbency, and preparation is like other techniques. Credie35 used a convex transducer in dogs, positioned transversely and then sagittally over the lumbosacral region, allowing visualization of the contained structures in Figures 16 and Figure 17. The needle is precisely inserted, avoiding angle changes and potential injuries.36
In felines, anatomical landmarks are harder to identify due to their smaller size, making techniques like ultrasound especially advantageous. Studies by Credie and Luna37 and De Lima et al.38 demonstrated the efficacy of ultrasound in accurately identifying the anatomy of the lumbosacral epidural space in cats (Figure 18). Credie39 https://pubmed.ncbi.nlm.nih.gov/35091049/ concluded that ultrasound facilitates epidural anesthesia in animals with unfavorable body conditions, reducing the number of attempts, bone contacts, and vessel punctures (Figure 16), (Figure 17), (Figure 18).

Figure 16 Cross-sectional ultrasonic beam.
Source: Credie.39
Legend: Incidence of the ultrasonic beam on the lumbosacral space in cross-section.

Figure 17 Ultrasonic beam in oblique sagittal and parasagittal section.
Source: Credie.39
Legend: Incidence of the oblique sagittal and parasagittal ultrasonic beam on the vertebra.
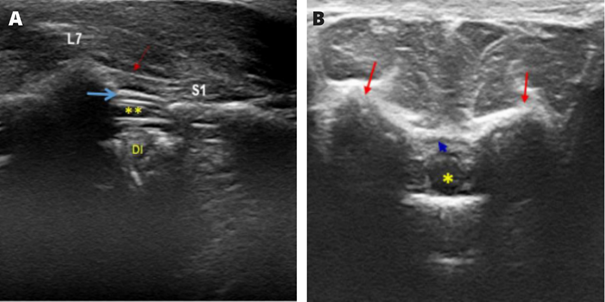
Figure 18 Sonoanatomy of the epidural space in felines.
Source: De Lima et al.40
Caption: (A) Ultrasound image of the structures in the sagittal plane: supraspinatus ligament (red arrow); epidural space (blue arrow); dural sac (**); DI, intervertebral disc; L7, spinous process of the seventh lumbar vertebra; and S1, the first sacral vertebra.
(B) Ultrasonographic image of the structures in the transverse plane: dural sac; iliac wings (red arrows), and epidural space (blue arrow).
Continuous epidural anesthesia
The use of an epidural catheter facilitates continuous drug administration in the epidural space, offering several advantages. These include adjusting local anesthetic volume based on each animal's needs, assessing the patient's response to diluted drug concentrations, redosing in long-term surgeries, and maintaining analgesia in postoperative and chronic pain scenarios.40
For this technique, the patient should be in sternal decubitus, and trichotomy and antisepsis are necessary. Following puncture and correct needle placement in the epidural space (using previously described techniques), a small initial dose of local anesthetic can be administered to ease catheter insertion. An adapter may be attached to the needle to provide rigidity to the catheter (Figure 19).4

Figure 19 Epidural catheter.
Source: Otero and Campoy.4
Caption: Note that the catheter is coiled and is held to prevent it from falling out of the sterile area.
The catheter should be cranially inserted through the Tuohy needle, advancing without resistance and not exceeding 3 cm to reduce the likelihood of knots and entry into an intervertebral foramen. Once positioned, the needle is carefully removed, ensuring the catheter tip is near the target nerve roots. The catheter should be held at its entry point when withdrawing the needle to prevent its inadvertent removal (Figure 20). If difficulties arise or the catheter needs removal, it should not be pulled out alone due to the risk of rupture within the epidural space.4,10,17
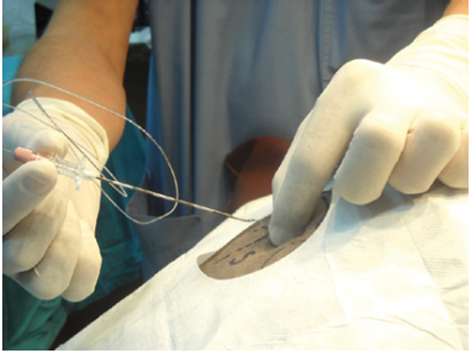
Figure 20 Removal of the needle in an epidural catheter.
Source: Otero and Campoy.4
Caption: After the catheter is in position, the needle should be withdrawn over the catheter. When the needle is withdrawn, the anaesthetist should hold the catheter at its point of entry to prevent its inadvertent removal with the needle.
According to Otero and Campoy,4 for catheter fixation, subcutaneous tunneling (Figure 21) should be performed several centimeters from its point of entry to minimize the potential for contamination. After confirming the absence of spontaneous flow of CSF or blood into the catheter, it can now be fixed using adhesive devices.
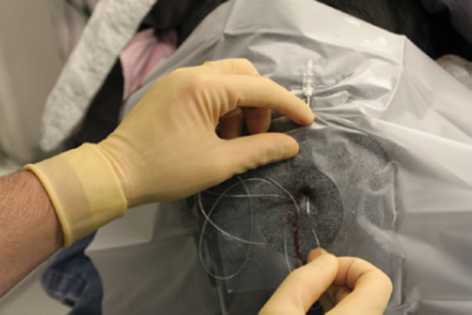
Figure 21 Epidural catheter tunneling.
Source: Otero and Campoy.4
Caption: Subcutaneous catheter tunneling several centimeters from its point of entry provides stable fixation while minimizing potential contamination of the catheter site.
Epidural doses and volume
The anesthetic volume in the epidural space significantly impacts the quality of anesthesia. Common dosing methods for epidural local anesthetics are based on a volume-to-weight ratio (mL/kg) or the spine's length from the occipital condyle to the first coccygeal vertebra (mL/cm).17,37 Futema10 suggests drug protocols and doses in mg/kg, tailored to the procedure, for both lumbosacral and sacrococcygeal epidural (Table 6).
|
Surgeries in the perineal region |
Orthopedic Surgeries of pelvic limbs in dogs |
|
- 2% lidocaine 4mg/kg + Fentanyl 1mg/kg |
- 2% lidocaine 4.5mg/kg + Fentanyl 1.5mg/kg |
|
- 2% lidocaine 2mg/kg + 0.5% bupivacaine |
- 2% lidocaine 3mg/kg + 0.5% bupivacaine 0.6mg/kg + fentanyl 1.5mg/kg |
|
- 0.5% Bupivacaine 0.5mg/kg + Fentanyl 1mg/kg |
- 0.5% Bupivacaine 1mg/kg + Fentanyl 1.5mg/kg |
|
- 0.5% bupivacaine 1mg/kg + Sufentanil 1mg/kg |
|
|
Abdominal surgeries |
Sacrococcygeal or sacrocaudal epidural in felines |
|
- 2% lidocaine 6mg/kg + Fentanyl 2mg/kg |
· Obstructed Cat Management |
|
- 2% lidocaine 4mg/kg + 0.5% bupivacaine 0.7mg/kg + Fentanyl 1.5mg/kg |
- 2% lidocaine 4mg/kg |
|
- 0.5% Bupivacaine 1.5mg/kg + Fentanyl 1.5mg/kg |
· Orthopedic surgeries of pelvic limbs |
|
- 0.5% bupivacaine 1.5mg/kg + Sufentanil 1mg/kg |
- 2% lidocaine 8mg/kg + Fentanyl 1mg/kg |
|
- 2% lidocaine 4mg/kg + 0.5% bupivacaine 1mg/kg + Fentanyl 1.5mg/kg |
|
|
|
- 0.5% Bupivacaine 1.5mg/kg + Fentanyl 1.5mg/kg |
Table 6 Suggestion of protocols and doses for epidural
Note: Morphine can be added to all protocols at a dose of 0.07 to 0.1mg/kg (10mg/mL concentration)
Source: Futema.10
A study using methylene blue dye in the epidural space of felines established a linear relationship between the volume and cranially affected vertebrae. The results indicated that 0.1 mL/kg reached up to L3 and L4, 0.2 mL/kg to L1 and L2, 0.3 mL/kg to T7-T11, and 0.4 mL/kg to T6-T10. The study noted a tendency for cranial migration of the dye due to the sternal decubitus positioning.38 Lee et al, suggest that 0.2 mL/kg volumes of local anesthetic can prevent cranial spread beyond the thoracolumbar region in cats. In dogs, according to Valverde,3 0.26 mL/kg of methylene blue reached T11-T13. For drugs like opioids that don't cause sympathetic or motor blockade, strict adherence to this volume rule may be unnecessary.
Larger anesthetic volumes aimed at reaching cranial spine segments can block numerous sympathetic fibers along the trunk, potentially causing hypotension and motor fiber blockage, affecting diaphragm and respiratory function. Other complications include transient spinal cord compression discomfort.3
Surgical applications
The administration of various drug combinations depends on the type of surgery. For perineal region surgeries in dogs, combinations of 2% lidocaine with fentanyl or 0.5% bupivacaine are used, while for orthopedic surgeries of the pelvic limbs, higher doses of lidocaine and fentanyl are suggested. In abdominal surgeries, increased doses of lidocaine, bupivacaine, and fentanyl are recommended. For sacrococcygeal or sacrocaudal epidural in felines, protocols vary from lidocaine alone to combinations with bupivacaine and fentanyl. Morphine can be added to all protocols at a dose of 0.07 to 1mg/kg.10
Study on lumbar epidural
The study "Lumbar Epidural: Anatomical and Clinical Study in Dogs Submitted to Ovariohysterectomy" by Cima et al.39 compared the dispersion of 0.25% bupivacaine with iohexol via lumbar epidural under fluoroscopic guidance in cadavers and live dogs. It aimed to evaluate postoperative analgesic consumption and sedation for 6 hours post-extubation in dogs undergoing ovariohysterectomy. The results indicated greater epidural dispersion in live dogs compared to cadavers and highlighted that all dogs treated with fentanyl and only one with 0.25% bupivacaine required postoperative rescue analgesia. Sedation scores were higher postoperatively in both groups. The study concluded that lumbar epidural anesthesia improved postoperative analgesia and prolonged sedation compared to fentanyl use.39
This study has demonstrated that the epidural anesthesia technique effectively provides analgesia during both transoperative and postoperative periods when applied as described in existing literature. While the procedure of epidural anesthesia is relatively straightforward, it necessitates veterinarians to have a sound understanding of technical aspects and the ability to accurately identify anatomical landmarks.
Over the past two decades, there has been significant advancement in the methods used to locate the epidural space, particularly with the advent of ultrasonography. Most referenced studies in this manuscript have focused on epidural approaches to the caudal segments of the spine, predominantly using lidocaine and bupivacaine, often in combination with opioids.
Recent research, including studies by Credie41 and De Lima et al.,40 The literature predominantly focuses on the lumbosacral and sacrococcygeal regions for dogs and cats, respectively, due to their anatomical specificities. However, recent research by Cima demonstrated that higher punctures are possible and recommended, decreasing the volume of anesthetic and side effects. It is essential to consider the administered volume, as there is a direct correlation between volume and potential adverse effects.
The limited frequency of thoracic surgeries in veterinary practice also contributes to the scarcity of studies on epidural anesthesia in this area.42
Drawing parallels with human medicine, where cranial spinal segment punctures are routine in anesthetic practice,40 advancements in veterinary technical knowledge are expected to facilitate similar approaches, especially as surgeries requiring thoracic epidural become more common.
In conclusion, while epidural anesthesia is generally safe, it is not without potential complications and side effects, akin to any anesthetic procedure. Each case must be evaluated individually, prioritizing the patient's well-being.
None.
Author declares there are no conflicts of interests.
None.

©2024 Serrano, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.