Journal of
eISSN: 2373-4345


Research Article Volume 10 Issue 1
1Department of Endodontics, Faculty of Dentistry, Norbert Wiener University, Peru
2Department of Endodontics, Faculty of Dentistry, UPC, Peru
3Department of Dentistry, State University Ponta Grossa, Brazil
Correspondence: John Alexis Dominguez, Department of Endodontics, Faculty of Dentistry, Norbert Wiener University, Arequipa 440,Lima, Peru, Tel 51949725703
Received: January 11, 2019 | Published: January 23, 2019
Citation: Macedo LPC, Riquero RN, Falla RV, et al. Ultrasonic removal efficacy of calcium hydroxide inside root canal walls with different power rating and activation time protocols. J Dent Health Oral Disord Ther. 2019;10(1):59-62. DOI: 10.15406/jdhodt.2019.10.00460
The objective of this study is to evaluate the ultrasonic efficiency to remove calcium hydroxide from the root canal walls, with different power rating and activation time protocols. Material and Methods: Extracted single rooted premolars were instrumented with Twisted File #40/.04. Each canal was filled with Ca(OH)2, and all specimens were stored in 37ºC at 100% relative humidity for 1 week. The specimens were aleatory distributed in to nine experimental groups (n=20), as follows: G10x10; G10x20; G10x30; G20x10; G20x20; G20x30; G30x10; G30x20; G30x30; where the first value corresponds to the power rating (in %), and the second value corresponds to the activation time (in s) of the ultrasonic tip (Irrisonic, Helse). The specimens were observed under a scanning electron microscope and the Ca(OH)2 amount was calculated by scores. Data were statistically analyzed by 2-way ANOVA and Tukey post-test. Results: G20X20 and G30X30 were significantly more efficient to remove Ca(OH)2 than the other groups (p<0.05), without significant differences between them. Conclusion: 20% of power rating with 20 seconds, and 30% of power rating with 30 seconds were more efficient to remove Ca(OH)2 from the root canal walls.
Keywords: calcium hydroxide, irrigation, removal, ultrasonic
The main objectives in endodontic therapy are disinfection and complete obturation of the root canal system.1 The disinfection may be obtained by chemo-mechanical preparation; however, clinical circumstances require the use of intracanal medication (ICM) when the endodontic treatment is performed in more than one session.2 Calcium hydroxide (Ca(OH)2) is the most commonly used intracanal medication (ICM) due to its antibacterial efficiency3,4 and biocompatibility.5 However, Ca(OH)2 accelerates the setting time of endodontic cements and sealers that are used to obturate the canals. This is probably due to inadequate Ca(OH)2 removal from the canal walls,6 which obliterate dentinal tubules7 and would result in poor-quality obturation.8 Authors also evaluated the microleakage in root canal walls medicated with and without Ca(OH)2, and they concluded that the ones which received ICM demonstrated the highest microleakage scores.9
One study evaluated the use of passive ultrasonic irrigation (PUI) to remove Ca(OH)2 in the root apical third, compared to conventional irrigation needle delivery. The results showed that PUI was significantly superior than the conventional needle delivery system.10 When comparing the needle delivery technique to passive ultrasonic activation and negative pressure (i.e.-EndoVac), better results were attributed to EndoVac. Similar results were obtained with constant, passive ultrasonic activation for one minute.11 However, in the mentioned studies they do not have a standardized time and power ratings protocol for the use of ultrasonic passive irrigation, which makes it difficult to compare them and establish an adequate protocol to use by clinicians. Considering the current controversial results in the literature regarding adequate root canal disinfection protocol, the objective of this study is to evaluate the ultrasonic removal efficiency of Ca(OH)2 along the root canal walls using different ultrasonic activation times and power ratings.
The protocol was approved by the Ethics Committee Norbert Wiener Private University. Single rooted premolars were extracted from patients at the University Norbert Wiener Private University (Lima-Perú), ultrasonically cleaned and stored in distilled water at 4°C. They were used within six months after their extraction. Teeth that exhibited clinical signs of dental caries, root resorption, cracks, or fractures were excluded, and they were radiographed to confirm their single-rooted anatomy. Additionally, using a diamond saw (MDT, Afula, Israel) under constant irrigation each sample was decoronated in order to obtain a standardized root length of 15±2mm from the apex.
The patency of the canals was verified by a 15 K-file (SybronEndo, Orange, California, USA) passing through the apical foramen, and the working length was determined to be 1 mm short of the total length of each tooth. A crown-down instrumentation technique (Twisted Files, SybronEndo, Orange, California, USA) was implemented in each sample tooth by enlarging the canal until 40.04 file, according to the manufacturer's instructions, in a reciprocating motion (VDW Silver Reciproc handpiece, VDW, Germany). The root canals were irrigated between each rotary file using NaviTip® (Ultradent Products, South Jordan, UT, USA) positioned 1 mm short of the working length with 1.0 ml of a 5.25% NaOCl and 1.0 ml of 17% EDTA solutions. After drying the canals with paper points, each canal was filled with Ca(OH)2 (UltraCal XS, Ultradent Products, South Jordan, UT, USA), except the negative controls. The presence of Ca(OH)2 in the root canals was confirmed with mesiodistal and buccolingual radiographs. The root access was temporarily filled with Coltosol® F (Coltene, Altstätten, Swiss). The specimens were stored for 7 days at 37˚C and 100% humidity.
After the storage period, the temporary restoration was removed, and the specimens were randomized in three experimental groups (n=20), according to the power rating and activation time of the ultrasonic tip used to remove the medication: G10x10; G10x20; G10x30; G20x10; G20x20; G20x30; G30x10; G30x20; G30x30. The first value corresponds to the power rating (in%), and the second value corresponds to the activation time (in s) of the ultrasonic tip. Each group had one specimen for positive control (no Ca(OH)2 was removed), and one specimen for negative control (the canal was completely filled by Ca(OH)2). In all protocols, the roots were irrigated with 1 ml of 5.25% NaOCl. The working length was checked (Twisted Files, 40/.04), and the ultrasonic tip (Irrissonic, Helse, Santa Rosa de Viterbo, SP, Brazil) was used with the ultrasonic unit VDW.ULTRA® (VDW, Germany) to continuously irrigate the canals with 3.0ml of 5.25% NaOCl, 2mm short of the working length. During activation, the needle was passively moved in an up-and-down motion to ensure it did not bind to the root canal wall. A final rinse with 1.0 ml of 17% EDTA and 3.0ml 0.5% NaOCl ended the irrigation protocol. After irrigation protocols, the specimens were longitudinally sectioned in a buccal and lingual direction with a diamond saw (Medin, Vlachovická, Czech Republic), and care was taken to avoid contact between the saw and the root canal surface. The specimens were separated into halves with a chisel.
Scanning Electron Microscopy (SEM)
The specimens were dehydrated for 48 hours in a desiccator (Dry Keeper Simulate Corp., Tokyo, Japan), and sputter coated with a 10 nm platinum layer (Polaron Equipment Ltd., Hertfordshire, England, UK). SEM analyzes were performed under 1000 X magnification in the middle third. The root canal wall area was calculated in order to evaluate the residual Ca(OH)2 amount. Using the scoring system previously described by Yücel11 and Alturaki,12 three calibrated investigators made the evaluations. Each canal was scored as follows:
Score 1: 80-100% of Ca(OH)2 removal: dentinal tubules were considered totally cleaned; dentinal tubules inside root walls may be totally visualized without the presence of any Ca(OH)2 amount (Figure 1A).
Score 2: 60-80% Ca(OH)2 removal: adequate cleaned; dentinal tubules inside root walls may be visualized, and there is minimal Ca(OH)2 amount on the canal walls (Figure 1B).
Score 3: 40-60% Ca(OH)2 removal: partially cleaned, and at least 50% of the dentinal tubules surface was covered with Ca(OH)2 (Figure 1C).
Score 4: 20-40% Ca(OH)2 removal: was considered poorly cleaned, the dentinal tubules along the canal walls may not be visualized, and there is a heavy presence of Ca(OH)2 amount (Figure 1D).
Score 5: 0-20% Ca(OH)2 removal: not cleaned, dentinal tubules inside the root canal walls are totally obliterated by the Ca(OH)2 medication (Figure 1E).
The mean scores were used for statistical purposes. Data was statistically analyzed by 2-way ANOVA and Tukey post-test (α=0.05).

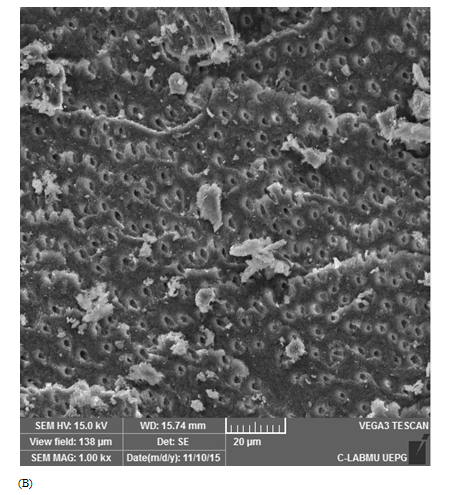
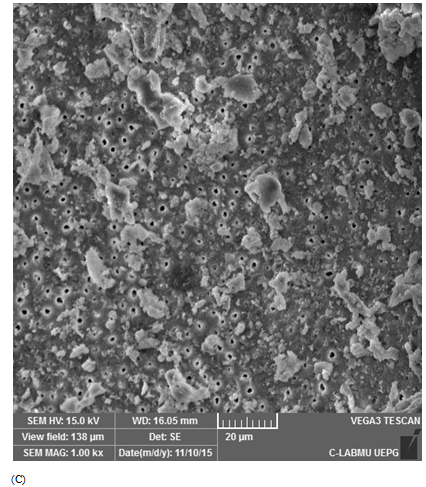
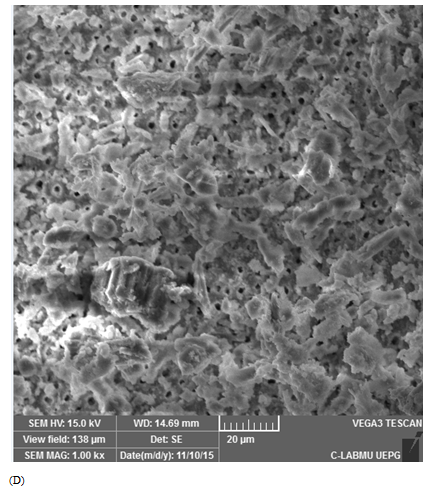

Figure 1 Scanning electron microscopic images representative of score attribution. (A) score 1: 80%–100% Ca(OH)2 removal (totally cleaned); (B) score 2: 60%–80% Ca(OH)2 removal (adequate cleanliness); (C) score 3: 40%–60% of Ca(OH)2 removal (partial cleanliness); (D) score 4: 20%–40% of Ca(OH)2 removal (poorly cleanliness), and (E) score 5: 0%–20% of Ca(OH)2 removal (not cleaned).
Positive controls demonstrated score 5, with dentinal tubules completely covered by Ca(OH)2 (Figure 1E). For the negative controls, score 1 was attributed for all specimens (Figure 1A). Table 1 describes mean and standard deviation of the obtained scores evaluated by SEM. The lowest scores were observed when the ultrasonic tip was used with a 20% power rating for 20 seconds (1.93±1.09) and with a 30% power rating for 30 seconds (2.58±1.33), being statistically different from the other groups (p<0.05). The other groups did not show any differences between them.
|
|
10 s |
20 s |
30 s |
|
10% |
4.08±1.12B |
3.61±1.27B |
4.11±1.07B |
|
20% |
4.41±0.78B |
1.93±1.09A |
3.29±1.24B |
|
30% |
3.67±0.87B |
4.34±0.85B |
2.85±1.33A |
Table 1 Mean and standard deviation of the scores obtained in each experimental group*
*Distinct letters demonstrated statistically significant differences (p<0.05)
The presence of Ca(OH)2 may influence the physical properties of sealers such as working time and set thickness.13 Also, there is a significant reduction in the bond strength of resin-based sealers due to Ca(OH)2 amount which covers the dentin/collagen network that is necessary for the resin adherence.14 Goldberg evaluated how Ca(OH)2 obliterate lateral canals hindering the obturation with the sealer. The results revealed that the negative control group (with no Ca(OH)2 amount) had a significantly higher number of adequately obturated lateral canals;15 therefore, it seems important to remove as much Ca(OH)2 as possible from the root canal before the obturation.
Among several irrigating protocols, the frequency of the early ultrasonic units ranged from 25 to 40 kHz. Irrigation frequencies are currently recommended to be used at a higher frequency than that of human hearing or 20 kHz.16 Ultrasonics has been widely used in the cleaning of the root canal. Authors have compared the debris removal efficacy between the manual and rotatory preparation with and without ultrasonic activation. An ex-vivo study histologically analyzed the extracted teeth and concluded that the ultrasonic activation improved cleaning efficacy in mesial roots of lower molars.17 The use of the scanning electron microscope and score scale, used in this study, to evaluate the Ca(OH)2 removal from the root canal walls, has been described in previous studies such Yücel11 and Alturaki.12 In these respective studies, the authors evaluated different final irrigation techniques and demonstrated that no irrigating protocols were able to completely remove the Ca(OH)2 medication from the root canal walls, which is similar to the results of the present study. The same results found for negative and positive controls were found in the present study, where positive control were totally obliterated by the Ca(OH)2 medication and negative control were totally visualized without the presence of any Ca(OH)2 amount.11,12
Other study showed that rotatory instrumentation combined with passive ultrasonic activation (3-20 s sessions) demonstrated small Ca(OH)2 amounts when compared to sonic irrigation.18 In the present study, the lowest scores were observed when the ultrasonic tip was used 3 times during 20 s. In other study, the following protocols were studied: passive ultrasonic irrigation, the common irrigating syringe, EndoVac and self-adjustable files to remove Ca(OH)2. Better results were found when the irrigants were continuously agitated using a #15 K-file coupled to the file-holding adapted handpiece and when the ultrasonic tip was activated at the sixth power setting of power setting available in that unit, as well as with the self-adjustable files.19
During this study, it was only evaluated the amount of Ca(OH)2 in the middle third. Other studies demonstrated no benefit in the passive irrigation used to remove Ca(OH)2 from the apical third. Comparing two techniques (manual and passive ultrasonic irrigation), and using four different methods to prepare the Ca(OH)2 mix, researchers found a significant Ca(OH)2 removal using passive irrigation in the cervical and middle thirds of the canal. However, they found no differences in the apical third.20
The improved irrigation of ultrasonic tips is due in part to cavitation, which is defined as the formation of small bubbles that are created and rapid explode. This explosion may promotes a cleansing "shock wave" within the irrigation solution, which produces a forceful energy that aids in debris removal from the instrumented canal walls.21
There is no consensus in the literature regarding a specific protocol when using ultrasonic tips in root canal spaces. Moreover, the duration of the required activation time is not clear. Researchers found higher debris removal with a 5-min activation when compared to 3 min,22 however, no differences were found in another study which used ultrasonic irrigation with 30 and 60 s of activation.23
Higher power setting does not necessarily produce more effective Ca(OH)2 removal, and this may be due to the fact that when power is increased, the cavitation bubbles that are produced may not successfully perform the mechanical action in which they are intended. Also, it was found that although the experimental group using a 30% power for 30 s successfully removed Ca(OH)2, this group showed micro-fractures within the dentinal tubules, which is confirmed by other study24 that the use of prolonged activation times may result in microfractures of the root canal walls. Consideration should be given in this study for the fact that the teeth were decoronated, which causes a loss of the irrigation solution coronally, thus, decreasing the hydrostatic pressure towards the ápex.12,18
Under the aspects of this study, none of the groups demonstrated complete removal of Ca(OH)2 from the root canal walls. However, the use of an ultrasonic tip with 20% power setting for 20 s, or the use of 30% power setting for 30s were more efficient to remove Ca(OH)2 from the canal walls.
None.
The author declares that there is no conflict of interest.

©2019 Macedo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.