Journal of
eISSN: 2373-4345


Research Article Volume 6 Issue 5
1School of Dentistry, Shiraz University of Medical Sciences, Iran
2Department of Microbiology, Jahrom University of Medical Sciences, Iran
Correspondence: Farzan Modaresi, Department of Microbiology, School of Medicine, Jahrom University of Medical Sciences, Motahari Boulevard Jahrom, Iran, Tel 989000000000
Received: March 05, 2017 | Published: March 10, 2017
Citation: Afra SM, Modaresi F. The use of synergistically antiplaque nanoparticles in treating dental caries. J Dent Health Oral Disord Ther. 2017;6(5):144-149. DOI: 10.15406/jdhodt.2017.06.00214
Nanoparticles have been suggested as useful antibacterial and anti-plaque solutions for children with dental caries. The purpose of this study was to compare the antimicrobial and antiplaque effects of chlorhexidine with Zinc and copper nanoparticles. Furthermore, considering the synergistically antibacterial and anti-biofilm effects of nanoparticles in clinical approaches and standard strains of Streptococcus mutans and Streptococcus sanguinis. The specimens were collected from 66 children between 3-5 years of age and detected S. mutansand S. sanguinis by PCR thereafter the antimicrobial activity of the chlorhexidine with Zinc and copper nanoparticles was measured by micro dilution and disc diffusion tests and colonies were counted after one to five minutes. The anti-plaque activity was examined by microtitre test. Use of alone nanoparticles showed higher minimum inhibitory concentration (MIC) than using them as synergistically. The best synergistically solution containing Zinc and copper nanoparticles demonstrated 0.039 μg/ml in S. mutans and S. sanguinis. Furthermore this solution had a lowest biofilm inhibitory concentration (BIC) and counted colony number than chlorhexidine. Solution containing Zinc and copper nanoparticles showed the lowest inhibitory and anti-biofilm concentration against S. mutansand S. sanguinis compared to those of other nanoparticle alone and chlorhexidine thus it may be to use as an alternative for people/cases with dental caries, dental plaques or oral infections.
Keywords: Streptococcus mutans; Streptococcus sanguinis; Nanoparticles; Anti-plaque; Antimicrobial; Dental caries
Dental caries is one of the world’s major public health problems. It is a multifactorial disease caused by low pH for a prolonged time within plaque, leading the enamel to dissolve.1 Dental plaque is a natural biofilm that consists of various bacterial species and extracellular matrix with soluble and insoluble glucans. It is affected by numerous external factors such as diet, saliva composition, and salivary flow rate.2 Acidogenic bacteria such as Streptococcus mutans, Streptococcus sanguinis and Lactobacilli are considered as the contributory factors of dental caries. Different bacterial species exist in a biofilm in close proximity to each other. Streptococci are the major colonizers of early enamel biofilms. Mechanical methods such as brushing teeth are impressive for plaque removal, but they are directly dependent on individual skills and are problematic in disabled or traumatized patients. The use of adjunctive methods such as mouthwashes has been shown to be effective for prevention of plaque accumulation.3 Routine mouth rinses like chlorhexidine, however, are associated with the disadvantages including enamel staining, taste disturbances and mucosal irritation.4 The antibiotics such as penicillin, cephalosporins and vancomycin are effective against corponding bacteria but side effects and highly antibiotic resistance have been observed. Therefore, searching for an alternative antimicrobial agent with minimal side effects seems to be highly demanding. Metal nanoparticles have long been used in medicine because of their bactericidal and bacteriostatic effects.5,6 The antibacterial properties of metal ions depend on their surface contact area. Decreased size of nanoparticles (<100 nm in diameter) results in an increased surface area and thus increases the interactions with organic and inorganic molecules. Several studies have shown that Zinc and copper nanoparticles are able to make structural changes in the cell membrane.7 Nano-sized ZnO exhibits varying morphologies and shows significant antibacterial activity over a wide spectrum of bacterial species explored by researchers.8 Interestingly, ZnO-NPs are reported by several studies as non-toxic to human cells. The various antibacterial mechanisms of nanomaterials are mostly attributed to their high specific surface area-to-volume ratios,9 and their distinctive physicochemical properties. However, the precise mechanisms are yet under debate, although several proposed ones are suggested and adopted. Copper has bactericidal action, mainly related to its ability to give and accept electrons in a continuous process.10 To date, there are only few studies that have determined the antimicrobial effects of nanoparticles against cariogenic and periodontal disease bacteria in simulated oral conditions.11 Furthermore, the synergistic effect of nanoparticles is not evaluated so much. This study aims to compare the antimicrobial and antiplaque effects of chlorhexidine with Zinc and copper nanoparticles and also considering the synergistically antibacterial and anti-plaque effects of nanoparticles in clinical and standard strains of S. mutansand S. sanguinis.
Sample size
Ghasempour et al.12 in 2013 reported prevalence of dental caries with S. mutans was to be 75.6% among preschool children with different levels of caries activity in Iran and Martinez et al.13 in 2012 reported prevalence of dental caries with S. mutans and S. sanguinis was to be 70% in preschool Mexican children. So for the present study prevalence of dental caries with involved bacteria was considered as 73%. Finally, the minimum sample size required using this prevalence at 95% level of confidence and desired level of absolute precision of 8% was calculated to be 118. So after meeting the inclusion and exclusion criteria a final sample of 66 were included for the study.
Patients’ clinical conditions and bacterial sources
66 Iranian pre-school children aged 3-5 years old, who were referred to Shiraz dental faculty, enrolled to this study. Consent for participation was obtained from at least one of their parents before the study according to the ethical guidelines of the Declaration of Helsinki (1975). Samples were collected from patients with diagnosis of caries based on the scratch of a dental explorer in a cavitation on the tooth surface and by bite-wing radiographs. These criteria would eliminate the white spot lesion from consideration but would include, as caries, surface defects such as developmental grooves by an expert laboratory technician.14 All 66 specimens obtained from saliva and teeth of the patients with dental caries, non-stimulated saliva samples were collected with a sterile cotton swab from the sublingual area of the mouth until saturated. Samples from the teeth (plaque) were taken with a sterile toothpick; the toothpick was placed in each proximal site and then passed along the gingival margin into the next proximal site of both upper and lower jaws. Swab and toothpick samples were placed into separate 1.0-mL reduced transport fluid vials and further processed.15 Appropriate dilutions of saliva and plaque samples were plated onto MM10-sucrose agar.16 After 3 days of anaerobic incubation (85% N2, 10% CO2, and 5% H2), colonies presumed to be S. sanguinis were selected from MM10-sucrose agar based on their firm, adherent, star-shaped colony morphology.17,18 Discrete colonies were then isolated from subcultures and placed in the appropriate medium for the detection of hydrolysis of arginine and lack of fermentation of mannitol. The prototype strain of S. sanguinis (ATCC 10556) and S. mutans ATCC 25175 were used as controls.
Polymerase chain reaction (PCR)
All 66 isolates were analyzed by PCR for confirmation of the biochemical tests. PCRs were performed to detect the S. mutans and S. sanguinis using the primers pairs.19 These primers were 5’-GqaGCACCACAACATTGGGAAGCTCAGTT and 5’-GGAATGGCCGCTAAGTCAACAGGAT for S. mutans that produced a 433bp fragment and 5’-GGATAGTGGCTCAGGGCAGCCAGTT and 5’-GAACAGTTGCTGGACTTGCTTGTC for S. Sanguinis with an amplicon size of 313bp size (Fig 1). Specificity of the candidate primers sequences in the database was verified by blast analysis (http://www.ncbi.nlm.nih.gov/GenBank). The genomic DNA was isolated with the genomic isolation kit (Thermo scientific, Lithuania) as described by manufacturer. DNA ladder obtained from Cinnagen Co. (Tehran, Iran). Each PCR was performed with 1 µL DNA template, primer Forward and Reverse (20 pM) 0.7 µL, DNA 0.8 µL, master mix 8 µL, DDW 5.8 µL, and 3 U of LA Taq polymerase. DNA amplification was performed in a temperature gradient thermal cycler (Biometra-T gradient, Germany) with initial denaturation temperature of 94⁰C for 5 min, 30 cycles of 94⁰C each for 35 s, annealing temperature was 60⁰C for 35 s respectively followed by extension at 72 ⁰C for 40 s and the final extension step was set at 72⁰C for 5min.
Preparation of bacterial suspensions
Antimicrobial experiments were carried out with 66 clinical isolates, S. mutans (ATCC 25175) and S. sanguinis (ATCC 10556) obtained from Pasteur Institute (Tehran, Iran). They were sub cultured in 5% sheep’s blood agar. At first, 5-6 colonies from an overnight culture were diluted in brain heart infusion broth and were incubated in an aerobic environmental condition for 1-2 h at 35°C to reach the concentration of 1.5×108 CFU/ml. The colonies were then diluted with saline solution to a final concentration of 1.5×106 CFU/ml.
Preparation of nanoparticles
Nanoparticles including Zn, and Cu were purchased from Nanosany Co., Iran. According to the supplier, nanoparticles were more than 99% pure after ignition. The nanoparticles were added to a water based-solution in microbiology laboratory at the Faculty of microbiology and Virology of Shiraz University of Medical Sciences, Shiraz, Iran. The nanoparticles were characterized by ultraviolet-visible spectroscopy (Shimatzu, Japan) and further examined by a particle size analyzer (Zetasizer [Nano-zs], Germany) to find out their size distribution. Mean size of the nanoparticles ranged from 30 to 45 nm. Colloidal solutions containing nanoparticles were prepared with initial concentration of 20 ppm and were sterilized in gravity autoclave before antimicrobial tests. The synergistically effect of nanoparticles was measured with equal concentration (20 ppm) of each nanoparticles.
Antimicrobial test
The lowest concentration of each antimicrobial agent that inhibits the growth of the microorganisms being tested is known as the minimum inhibitory concentration (MIC) and is detected by the lack of turbidity matching with a negative control. Furthermore, the minimum bactericidal concentration (MBC) is defined as the lowest concentration of an agent killing the majority of bacterial inoculums. The MICs for the experimental solutions were assessed using a spectrophotometric micro-dilution method (SMM) and turbidity. For each strain a 96-well ELISA plate was used and solutions were colored with resazurin (Sigma, St Louis, MO, USA). To generate a check board, row 1 was filled with control solutions (chlorhexidine), and from 2 onwards, the experimental solutions. Row 8 was used to insure the viability of bacteria strains, sterility of experimental solutions, and sterility of the medium. Solutions were subjected to a series of dilutions, and inserted in columns 1 through 12. Wells from row 1 filled with 140 μl BHI (brain heart infusion agar), 50 μl of control solution and 10 μl of exponentially growing bacterial culture (about 108 colony-forming units/mL). The wells of row 2 were filled with 100 μl BHI, 100 μl of experimental solutions, and 10 μl of exponentially growing culture. The plate was incubated at 37 °C for 18 hours, and the absorbance of each well was determined using an automatic ELISA tray reader (READWELL PLATE) adjusted at 630 nm, before and after incubation. Then all wells were filled with the oxidation-reduction indicator resazurin to assure the true viability of antimicrobial activity.20 The MIC value was expressed as the lowest concentration inhibiting the bacterial growth. Minimum bactericidal concentrations (MBCs) were determined when aliquots from the MIC wells did not present visible bacterial growth on plates with BHI, and incubated at 37°C for 24h. The MBC is defined as the lowest concentration of antibacterial agent that kills microorganism. All tests were performed in triplicate. These tests were performed using Zn and Cu nanoparticles and 0.2% chlorhexidine as a control.
Bactericidal time effect
To determine the required time before initiating bactericidal effect, 50 ml of each test specimen was mixed with 50 ml of the bacterial suspensions (containing 5×103 colonies). After 1 and 5 min, it was cultured on blood agar. Following overnight incubation at 37°C, the remaining colonies were counted.21
Effects of nanoparticles on mature biofilm
Streptococcal strains, as well as the un-stimulated saliva, were grown as biofilms using polystyrene flat-bottomed micro-titer plates in TSB (tripticate soy broth) plus 1% sucrose for bacteria and at 1% sucrose for saliva. After 24 h of incubation at 37°C with 5% CO2, the planktonic-phase cells were gently removed and wells were washed with PBS (phosphate buffer solution) and filled with 200 μl two-fold dilutions of the nanoparticles in 30% TSB plus 1% sucrose, or 30% TSB plus 1% sucrose (control). Plates were incubated for 24 h at 37 °C at 5% CO2. Optical density was measured (600 nm) at time 0 and after incubation for 24 h by spectrophotometry using an ELISA reader (Eppendorf, Germany). The biofilm inhibitory concentration (BIC) was determined as the lowest concentration where no growth occurred in the supernatant fluid, confirmed by no increase in optical density compared with the initial reading. For the determination of the biofilm eradication concentration (BEC), samples of biofilms from the bottom of these wells were scraped by a metal loop, and spread on TSA plates and incubated for 48 h at 37°C at 5% CO2. The BEC value was determined as the lowest concentration at which no bacterial growth occurred on the TSA plates.22
Statistical Analysis
Measures of central tendency and dispersion were calculated for each of the variables studied. Analysis of one way variance (ANOVA) and Tukey’s post hoc tests were used to compare MIC and MBC among groups studied. A value of p<0.05 was considered statistically significant. The T-test was used for analysis of differences between clinical and ATCC strains. We used SPSS 17 software to analyze data.
Table 1 shows the distribution of S. mutansand S. sanguinisin different age groups. In all subjects, the prevalence was 75.8% and 60.6%, respectively; 15(22.7%) were positive for S. mutansalone, 5 (7.6%) were positive for S. sanguinis alone, 35 (53 %) were positive for both S. mutansand S. sanguinis and 11(16.7%) were negative for both S. mutans and S. sanguinis. The reliability of S. mutansand S. sanguinis isolates were determined by biochemical tests and confirmed by PCR (Figure 1). The size of amplicons were 434bp and 313bp for S. mutansand S. sanguinis, respectively. Table 2 presents the means and standard deviations (SD) of MICs and MBCs in study groups against S. mutansand S. sanguinis. The solution containing two nanoparticles namely Zn and Cu had the lowest MIC against S. mutansand S. sanguinis and showed the synergistic effect. Results suggested that the combined nanoparticles had a lower MICs and MBCs than each nanoparticles used alone. In children of 3, 4 and 5 years old it was demonstrated that all clinical isolates had lowest MIC upon exposure to solution containing Zn and Cu followed by the solution with Zn that had a lower MIC and MBC in comparison to other antibacterial solutions shown in Table 3. The number of colonies of S. mutansand S. sanguinis after 1 and 5 min of bacterial exposure to each colloidal solution is reported in Figure 2. Nano Fe3O4 showed the largest colony count after 1 and 5 min of bacterial exposure. We observed that as the same as above solution containing Zn and Cu had the lowest colony number after 5 min than other solutions. A value of 0.05% (v/v) of Zn and Cu solution was lowest concentration able to inhibit the bacterial growth both on the fluid supernatant of the streptococcal biofilms (BIC) and on the mature biofilm (BEC) against S. mutans and S. sanguinis (Figure 3).
S. mutans |
S. sanguis |
3 year |
4 Year |
5 Year |
Total |
+ |
- |
5 |
6 |
4 |
15 |
+ |
+ |
8 |
12 |
15 |
35 |
- |
+ |
1 |
3 |
1 |
5 |
- |
- |
5 |
3 |
3 |
11 |
Total |
19 |
24 |
23 |
66 |
Table 1 Distribution of S. mutans and S. sanguinis in different age groups
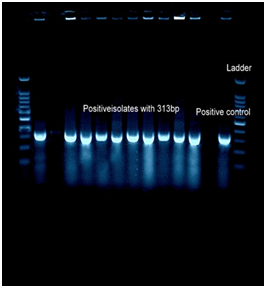

Figure 1 PCR amplification of S. mutans and S. sanguinis strains isolated in this study. The agarose gel electrophoresis (2%) was conducted at 90V for 90min. The gel was stained with 0.5µg/mL ethidium bromide and observed by UV gel documentation (UVITEC-Cambridge) system. Positive control S. sanguinis (ATCC 10556) and S. mutans ATCC 25175. A=313bp (S. sanguinis), B=433bp (S. mutans)
Group |
S. mutans (ATCC 25175) |
S. sanguis (ATCC10556) |
||
MIC |
MBC |
MIC |
MBC |
|
Nano Zn |
0.156 |
0.312 |
0.156 |
0.156 |
nano Cu |
0.156 |
0.312 |
0.312 |
0.312 |
Nano Zn+nano Cu |
0.039 |
0.078 |
0.039 |
0.156 |
Chlorhexidine |
50 |
50 |
25 |
50 |
Pvalue |
<0.05 |
<0.05 |
||
Table 2 Mean MIC and MBC (μg/ml) of the test groups against S. mutans and S. Sanguis
Group |
Number of Clinical Isolates with Lowest MIC |
||
3 years |
4 years |
5 years |
|
Nano Zn |
5 |
6 |
2 |
nano Cu |
4 |
3 |
1 |
Nano Zn+nano Cu |
10 |
15 |
20 |
Chlorhexidine |
0 |
0 |
0 |
Total clinical isolates |
19 |
24 |
23 |
Pvalue |
<0.05 |
<0.05 |
<0.05 |
Table 3 Frequency of clinical isolates with lowest MIC in different ages and test antimicrobial groups
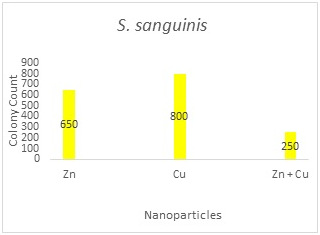
Figure 2A
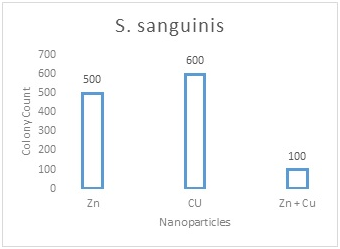
Figure 2B
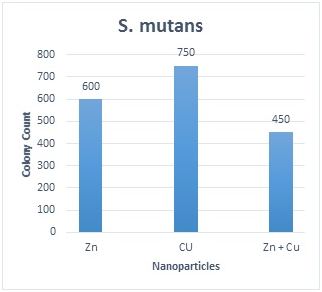
Figure 2C
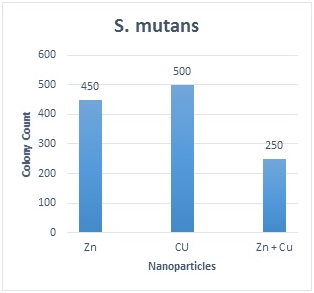
Figure 2D
Figure 2 The number of colonies of S. mutans and S. sanguinis after 1 and 5 min of exposure to each solution and the results of statistical analysis for comparison between groups. 2A: S. sanguinis after 1min; 2B: S. sanguinis after 5min; 2C: S. Mutans after 1min; 2D: S. mutans after 5min; Values represent the mean (±SD) of three independent experiments performed in duplicate.
In the present study the antimicrobial effects of chlorhexidine were compared with Zn and Cu nanoparticles and also considering the synergistically antibacterial and anti-biofilm effects of nanoparticles on clinical and standard strains of S.mutans and S. sanguinis. The antimicrobial activity of nanoparticles with approximately 30-40 nm was tested. Our results demonstrated the great synergistic effect of the solution containing Zn and Cu nanoparticles as an anticaries agent against S.mutans ATCC, S. sanguinisand clinical strains, because of its small MIC values. The artificial saliva was used in the test tubes to reveal any probable effect of proteins and other salivary constituents on the antibacterial activity of nanoparticle containing solutions. Antibacterial properties of some nanoparticles such as Zn and Cu have been evaluated in previous studies and different mechanisms have been proposed for their effects.23 Hernández-Sierra et al.24 indicated that zinc nanoparticle inhibits the growth of S. mutansat lower concentrations compared to nano Au and thus it may be more effective against dental caries.24 Zn nanoparticles show photocatalytic characteristics and prevent the accumulation of pathogenic bacteria. Most of the previous studies investigated the antibacterial properties of nanosilver and there is limited data available on the bactericidal properties of other nanoparticles. Combining nanoparticles may give rise to a more complete and complex bactericidal effect against a variety of bacterial populations.25 Furthermore, Mirhashemi al. obtained an average MIC of 5 µg/ml against S. mutansfor Zn nanoparticles which was three times more than our findings.26 This difference can be attributed to the method of disc diffusion test that was used to find the MIC by Mirhashemi and colleagues. The contact area of nanoparticles with bacterial microorganisms is higher in serial dilution method compared to the culture media, thus increasing their antibacterial effect. Sadeghi et al.27 evaluated the antimicrobial effect of chlorhexidine against S. sanguinis.27 They found an average MIC of 256 µg/ml, which was higher compared to the results of this present study. The antibacterial effect of nanoparticles was not desirable against S. mutans. It is possible that nanoparticles adhere together and form micrometer particles at high concentrations, which leads to less antimicrobial activity. In this study the MICs of nano Zn and nano-Cu was measured but interestingly it was confirmed that the combination of the nanoparticles had a lower MICs than the use them alone. The solution containing Zn and Cu was the most effective that this project proposed and had a lower antibacterial and anti-biofilm concentration activity. This may allow achieving better clinical effects with fewer side effects. Biofilm-related infections are difficult to treat because they are tolerant to the conventional chemotherapeutics, so innovative therapies are required to hinder their biofilm formation on restorative materials, and to avoid secondary caries. Furthermore this solution can reduce the number of S. sanguinis and S. mutansafter 1 and 5 min of bacterial exposure compared to other nanoparticle-containing solutions; however, the antibacterial effects of all the nanoparticle groups were significantly lower than that of the 0.2% chlorhexidine against S. mutansformation and to promote their potency. The solution containing Zn and Cu have a potential anti-biofilm capability to reduce biofilm, this property could be useful to prevent biofilm formation. This is in contrary to the results of Sadeghi et al.27 who showed that nano Zn had bactericidal effects against S. mutansafter 30 s, which was comparable to that of chlorhexidine.27 Due to increases of the antibiotic resistance in oral infection and the side effects of antibiotics in human organs, the oral infections need the new strategies in treatments. The potential of nanoparticles to control the formation of biofilms within the oral cavity, as a function of their biocidal, anti-adhesive, and delivery capabilities, is now coming under close scrutiny. In the present study, the scattered microdilution method (SMM) was used to determine the MICs of the test groups. This method is more accurate compared with the disc diffusion test and is more easily interpreted. It should be noted that complete simulation of the oral cavity is not possible under the laboratory conditions. The incubator cannot completely resemble the mouth. Furthermore, the antibacterial agents contact constantly with bacterial microorganisms in the culture media or test tubes, but the contents of mouthwashes are diluted and neutralized immediately in the oral cavity. Further studies are warranted to elucidate the antimicrobial effects of nanoparticle solutions when used as mouthwashes under in vivo conditions and any possible side effects of these solutions on oral microflora.
The solution containing nano Zn and nano-Cu showed the lowest inhibitory and anti-biofilm concentration against S. mutansand S. sanguinis compared to those of other Nanoparticles containing solutions and chlorhexidine thus it may be further investigated as an alternative to chlorhexidine.
None.
None.
The authors declare that there is no conflict of interest.

©2017 Afra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.