Journal of
eISSN: 2373-4345


Research Article Volume 13 Issue 3
1Università degli Studi di Torino, Dental School, Torino, Piemonte, Italy
2Division of Hematology, Università degli Studi di Torino, Torino, Piemonte, Italy
3Department of Economic and Business Science, Università di Cagliari, Sardegna, Italy
4Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129, Turin, Italy
Correspondence: Dr Tiziana Ruggiero, Oral Surgery Unit, Dentistry Section, Department of Surgical Sciences, University of Turin, Dental School, Via Nizza 230, 10100, Turin, Italy, Tel 39 334 9835933
Received: July 22, 2022 | Published: August 2, 2022
Citation: Ruggiero T, Bezzi M, Camisassa D, et al. Prevention and treatment of oral mucositis in patients undergoing hematopoietic cell transplantation with sodium hyaluronate and collagen precursors. J Dent Health Oral Disord Ther. 2022;13(3):54-58. DOI: 10.15406/jdhodt.2022.13.00572
Background: Oral mucositis is a frequent inflammatory complications of the oral mucosa in patients undergoing immunosuppressive therapy for hematopoietic cell transplantation; currently there is no standardized therapy. The sodium hyaluronate and amino acids (SH-AA) combination has been shown to be effective as a therapy in those patients. The aim of the study was to evaluate the clinical effects of SH-AA as a prevention in wound healing and pain management of oral mucositis.
Methods: A randomized experimental study was designed with a total of 74 patients, divided into two groups according to the treatment they were to undergo. Group A: received professional oral health treatment associated with therapy with SH-AA; Group B: received professional oral health treatment and were prescribed a standard treatment with Chlorhexidine 0.20%. The patients were examined and was recorded any appearance of oral mucositis and its severity (WHO scale), the number of lesions, their persistence and the number of days necessary for them to heal.
Results: Data obtained show patients of group A to have a lower risk both of developing mucositis (p = 0.005*) and of it occurring in a severe form, compared with group B (p = 0.003*), while those who developed it healed sooner (p = 0.01*).
Conclusions: In conclusion, this study proves the association between the SH-AA product use and a reduced incident and occurence of OM in patients undergoing HSCT.
Keywords: oral mucosa, wound healing, oral mucositis, sodium hyaluronate
Oral mucositis is one of the most debilitating and frequent inflammatory complications of the oral mucosa in patients undergoing immunosuppressive therapy for HCT (hematopoietic cell transplantation).1,2 The lesions can be so severe that they compromise the nutritional status of the patient or make it impossible to continue the therapy; commonly rinses of Clorexidine have been used for the treatment.3–6
Currently there is no standardized therapy for oral mucositis, but thanks to new knowledge about the complexity of the mechanisms underlying its etiology it has been possible to offer new therapeutic objectives.7,8 Prevention of oral mucositis has been investigated in the last MASCC Guide Lines.9 The difficulty in developing effective therapies is due to the need to combine the modulation of chemo- and radio-induced toxicity without decreasing the anti-tumour effect of the antineoplastic therapy.5,10–13
The sodium hyaluronate and amino acids (SH-AA) combination has been shown to be effective as a therapy in patients with oral mucositis. It is used in the form of a mouthwash and a spray. Both preparations contain sodium Hyaluronate (SH), combined with a pool of amino acids (AA) precursors of collagen, including L-Proline, L-Leucine, L-Lysine and Glycine, whose purpose is to assist the regenerative process of the oral mucosa to promote fast healing of the areas of ulceration and the simultaneous reduction of pain. SH, the main component of connective tissue and therefore strongly represented in the extracellular matrix of tissues, is known to be involved in fundamental physiological and pathological processes, including wound healing. It acts as a mucoadhesive polymer and as a barrier agent, inhibiting tissue destruction and promoting the healing process, relieving pain thanks to the prolonged contact of the product with the mucosa; and the precursor amino acids of collagen enable the production of this substance and of glycosaminoglycans by fibroblasts, which are key elements for tissue reconstitution during the healing process.14,15
In clinical practice, these properties result in improved epithelial repair in patients with various kinds of oral mucosal lesions. Owing to the properties of its components, this spray acts on the ulcerative phase of mucositis, creating a protective layer that reduces pain, especially in terms of immediate relief, and accelerates clinical healing. Clinical studies have demonstrated the effectiveness of reducing the number, extent and severity of lesions.16,17
But the characteristics of this compound probably also make it suitable as a preventive treatment. In this study differences between rinse of SH-AA and Clorexidine were evaluated in the prevention of oral mucositis and in reducing gravity when they occurred.
Purpose of the study
The primary outcome of the study is to evaluate if clinical effects of SH-AA rinses as a prevention in wound healing and pain management of oral mucositis during hematopoietic stem cell transplantation are better than Clorexidine rinses. Secondary outcome is to evaluate severity of OM healing times measured in days of oral mucositis occurrence in the two groups.
This randomized, controlled, single-blind, parallel design clinical trial was designed according to the CONSORT statement for improving the quality of reports of randomized controlled trials (http://www.consort-statement. org/) in Figure 1.
173 adult patients about to undergo an allogeneic or autologous cell transplant were recruited from the Department of Oral Surgery, Dental School, University of Turin, as they refer for evaluation and possible healing of the oral cavity, and subsequently assessed in the Haematology departments. Patients awaiting HCT underwent an extra and intra oral physical examination and radiological investigations to evaluate the need for dental treatment, compatibly with the timing of the transplant.
A randomized experimental study was designed with a total of 173 patients, and these were divided into two groups according to the treatment they were to undergo for the prevention of mucositis. A computer-generated randomization list was generated, and only one external investigator who not involved in the study was aware of its sequence and could have access to the file.
The inclusion criteria were: need for bone marrow transplant due to leukemia or blood dyscrasia; age over 18; adequate cognitive level; spontaneous participation in the study. The exclusion criteria were: age under 18; known allergy to one of the components of the compound; inadequate cognitive level; simultaneous presence of any mucocutaneous disease; presence of Graft Versus Host disease; severe deterioration of general condition of health; refusal to participate in the study.
The 74 patients selected were thus divided:
Group A: made up of 37 patients who received professional oral health treatment (a session with a dental hygienist including dental debrightment, root planing and scaling, motivation to maintain a correct oral hygiene at home) and were associated with therapy with SH-AA, administered from the first day of hospitalization for preventive purposes (7 days before transplant). SH-AA in the form of a mouthwash was administered by having the patient perform 3 rinses per day, held in the mouth for one minute without rinsing and without using water for 30 minutes after mouthwashing. In case of onset of lesions from oral mucositis the additional use was recommended of the spray form in the presence of symptoms, to be sprayed onto the lesions, up to 10 puffs per day. The spray was to be kept in place without rinsing or consuming water or meals for the following 30 minutes; it could be swallowed.
Group B: composed of 37 patients who underwent professional oral health treatment (a session with a dental hygienist including dental debrightment, root planing and scaling, motivation to maintain a correct oral hygiene at home) and were prescribed a standard treatment with Chlorhexidine 0.20%, administered from the first day of admission (7 days before the transplant) and for its duration. Chlorhexidine was administered by having the patient perform 2 rinses a day, kept in the mouth for one minute without rinsing and without using water for 30 minutes after application.
All patients were examined by an expert operator first before admission with a medical record being compiled and recorded, and all oral hygiene session were performed by the same dental hygienist, blinded to the therapy given during hospitalization. During first session the operator registered dental plaque index (P.I.) and bleeding index (B.I.) with a millimeter probe recording a periodontal chart.
All patients were then examined starting from the first day of hospitalization always by the same operator, a single oral health care provider blinded to the treatment who evaluated the clinical aspects of the lesions by recording their size and site of appearing, recorded any appearance of oral mucositis and its severity on the WHO scale, the number of lesions, their persistence within the oral cavity and the number of days necessary for them to heal. WHO mucositis scale ranging from 0= no symptoms; 1=soreness, erythema; 2=erythema, ulcers but able to eat solids,12 3=ulcers but required liquid diet; 4=oral alimentation not possible. We divided the lesions into two groups: light mucositis (WHO grade 1 and 2) and severe mucositis (WHO grade 3 and 4).
The present trial has been conducted in line with the principles of the Helsinki Declaration of 1975, as revised in 2000. This study was also accepted by Internal Review Board of our Centre. All subjects provided informed consent to the proposed study.
Calculation of the required sample size was done in order to determine the effect of interest with a power of 95% and a significance level of 5%.The value related to the success rate of treatment with Chlorexidine was taken from the study of Roopashri.18 Given these parameters it is necessary to enroll at least 32 subjects per group and, considering the possibility of a dropout that does not exceed 10%, it is decided to enroll 35 patients per treatment. The estimation of the sample size was performed using STATA/SE, version 14, software (StataCorp LP, College Station, TX, United States).
For the statistical analysis of the results, the R Commander Program and the following tests were used: Shapiro Wilk to determine the normality of the variables, Test F to evaluate the homogeneity of the variables; the T-Test, the Wilcoxon Test for independent data, the Chi-Square Test, Fisher and Anova Test for statistical significance. Continuous variables were expressed as mean and standard deviation and an error α = 5% (p-value = 0.05) was considered as the level of statistical significance.
The patients enrolled for the study are 74, divided into two groups of 37 each. In group A, 36 of 37 patients underwent intervention, and one was excluded from the analysis. In group B, 32 of 37 patients underwent intervention, 3 patients died during the study and 2 did not follow the indicated protocol correctly.
Patient groups were homogeneous in terms of age, gender and underlying diseases, also their oral conditions status, recorded with plaque index score and bleeding index score were comparable, as shown in Table 1.
A |
B |
||
Sex |
Mean |
23 |
19 |
F |
13 |
13 |
|
tot |
36 |
32 |
|
Age |
Mean |
51,6 |
52,9 |
Sd |
12,8 |
10,8 |
|
Plaque index |
Mean |
53,9 |
55,6 |
Sd |
32,4 |
24,6 |
|
Bleeding index |
Mean |
35,8 |
36,8 |
Sd |
30,6 |
24,5 |
Table 1 Patients’ baseline. Group A: patients who received professional oral health treatment associated with therapy with SH-AA; Group B: patients who underwent professional oral health treatment and were prescribed a standard treatment with Chlorhexidine 0.20%
The patients who developed mucositis, as seen in Figure 2, were:
22 out of 36 in group A (61.1%)
29 out of 32 in group B (90.6%)
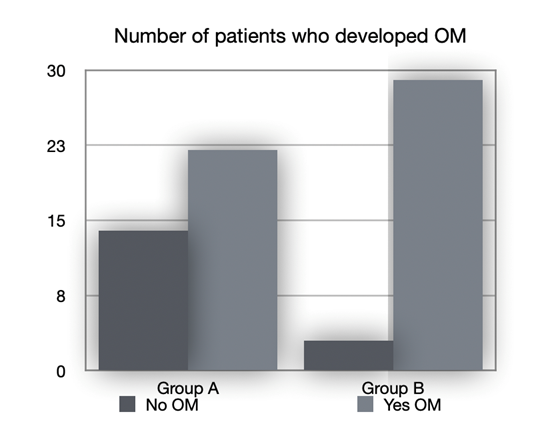
Figure 2 Number of patients who developed OM. No OM: patients who didn’t developed oral mucositis; Yes OM: patients who developed oral mucositis. Group A: patients who received professional oral health treatment associated with therapy with SH-AA; Group B: patients who underwent professional oral health treatment and were prescribed a standard treatment with Chlorhexidine 0.20%.
The difference between the number of mucositis lesions found in group B and those found in group A was statistically significant (p = 0.005*). These data demonstrate how the protocol, which involves carrying out a professional oral hygiene session in association with treatment with SH-AA from the first day of hospitalization, is protective against mucositis compared with protocols that envisage the sole use of Chlorhexidine in association with the professional oral hygiene session.
In group A, consisting of 36 patients for whom professional oral health treatment was performed, with an associated therapy with SH-AA, administered from the first day of hospitalization for preventive purposes, of the 22 mucositis found, 15 were classified as WHO 1-2, and 7 as WHO 3-4 (Figure 3).
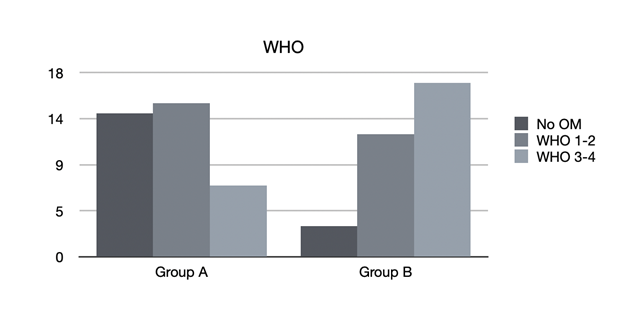
Figure 3 Mucositis classification. No OM: patients who didn’t developed oral mucositis; WHO grade 1 and 2: light mucositis; WHO grade 3 and 4: severe mucositis. Group A: patients who received professional oral health treatment associated with therapy with SH-AA; Group B: patients who underwent professional oral health treatment and were prescribed a standard treatment with Chlorhexidine 0.20%.
In group B, consisting of 32 patients who received professional oral health treatment and were prescribed standard treatment with Chlorhexidine 0.20%, of the 29 mucositis that were found, 12 were classified as WHO 1-2 and 17 WHO 3-4.
By comparing the groups, in relation to the severity of the mucositis (according to WHO scale), we obtained a statistically significant difference between group B and group A (p = 0.003*), demonstrating that not only were patients who underwent treatment with SH-AA less likely to experience mucositis, but also that, when this occurred, it was significantly less severe and disabling, compared with patients who did not undergo the treatment.
Finally, the healing times of the mucositis found in the various groups were evaluated (Figure 4).
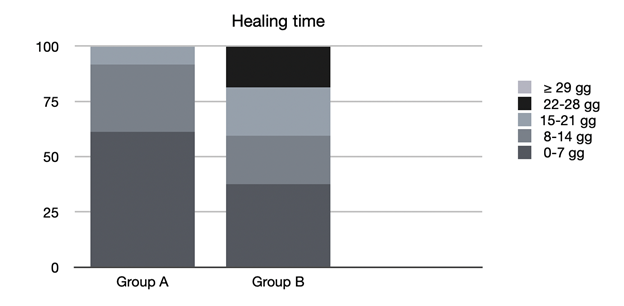
Figure 4 Healing times. D: days. Group A: patients who received professional oral health treatment associated with therapy with SH-AA; Group B: patients who underwent professional oral health treatment and were prescribed a standard treatment with Chlorhexidine 0.20%.
In group A, 61.1% of mucositis healed between 0 and 7 days, 30.6% between 8 and 14 days, 8.3% between 15 and 21 days and none after 22 days.
In group B, 37.5% of the mucositis healed between 0 and 7 days, 21.9% between 8 and 14 days, 21.9% between 15 and 21 days, 18.7% between 22 and 28 days and 0% in more than 29 days.
By again comparing the different treatments, we noted that the patients in group A enjoyed a faster healing of the lesions (all within 21 days, and 61.1% within the first week) compared with patients in groups B with statistically significant time difference (p=0.01*), demonstrating that treatment with SH-AA also plays a favourable role in the time of resolution of mucositis.
As can be seen from the results of the study, patients who underwent professional oral hygiene treatment and the administration of SH-AA from the first day of hospitalization for preventive purposes developed fewer cases of mucositis than patients who underwent oral hygiene and treatment with Chlorhexidine 0.20%. These were less severe on the WHO scale and achieved a significantly faster healing.
The protocol providing for the SH-AA and professional hygiene session performed by a dedicated operator, had a decisive effect on the appearance of mucositis, its severity and its duration over time.19,20
From the data obtained from this study it can be deduced that patients who have taken SH-AA from the first day of hospitalization for preventive purposes and have undergone an oral hygiene session have a lower risk both of developing mucositis, and of it occurring in a severe form, compared with those who have not taken it, while those who develop it heal sooner.
The use of SH-AA in both formulations, spray and mouthwash, therefore has a protective effect against the risk of mucositis, reducing its severity and the duration of the lesions associated with hematopoietic stem cell transplantation. This in addition to having a fundamental role in their treatment once they arise, as demonstrated by our previous study.21,22
The effectiveness of SH-AA in reducing pain and accelerating wound healing is due to its ability to repair tissues, activate and modulate the inflammatory response, promote cell proliferation and migration, angiogenesis, increase in re-epithelialization, and the deposition of new basal layers of keratinocytes and collagen, enabling the formation of a protective layer on the ulcerative layers.15
In conclusion, this study proves the association between the use of SH-AA in both formulations, spray and mouthwash, as a prevention and therapy of OM in patients undergoing HSCT. The sinergy of the association with professional oral health treatment shows a reducion of OM in incidence, severity, and duration of the mucositis after transplantation. The results of the present study encourage to continue the research and to further prospective research on the topic to confirm the effeciveness of the use of SH-AA in prevention and treatment of Oral Mucositis.
T Ruggiero: Conceptualization, Data curation, Writing-review & editing
M Bezzi: Writing-original draft; D. Camisassa: Formal analysis
R Pol: Investigation, Methodology, Supervision
L Giaccone: Resources
L Casula: Formal analysis, Methodology
S Carossa: Project administration
None.
The author declares no conflicts of interest.

©2022 Ruggiero, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.