
Research Article Volume 2 Issue 2
Microbiological analysis of teeth with chronic apical periodontitis and the outcome of treatment by gutta percha points impregnated with either calcium hydroxide or chlorhexidine as intra canal medicament
Marwa Sharaan
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Suez Canal University, Egypt
Correspondence: Marwa Sharaan, Suez Canal University, Ismailia, Egypt
Received: March 14, 2015 | Published: May 7, 2015
Citation: Sharaan M. Microbiological analysis of teeth with chronic apical periodontitis and the outcome of treatment by gutta percha points impregnated with either calcium hydroxide or chlorhexidine as intra canal medicament. J Dent Health Oral Disord Ther. 2015;2(2):68-77. DOI: 10.15406/jdhodt.2015.02.00046
Download PDF
Abstract
Microbiological analysis of teeth with chronic apical periodontitis and the outcome of treatment by gutta percha points impregnated with either calcium hydroxide or chlorhexidine as intra canal medicament Bacteria have a major role in the success of an endodontic treatment. Mechanical preparation alone cannot effectively eliminate bacteria from dentinal tubules and other irregularities in the root canal. Few remnant microorganisms can multiply between appointments often reaching the same level as at the start of the previous session. These observations call for an effective intracanal medication that will help to disinfect the root canal system. Calcium hydroxide has an antibacterial effect on most of the endodontic pathogens. This is due to its high alkalinity. It damages the bacterial cytoplasmic membrane. Chlorhexidine has been found to have a broad-spectrum antimicrobial action and relative absence of toxicity. Calcium hydroxide points as well as chlorhexidine gutta percha points are ready to be used in a flexible way and easily to be introduced into the canal. Three bacterial cultures - before, after instrumentation and after medication by either one of the experimental materials - were taken from forty healthy patients having non-vital single rooted teeth with chronic apical periodontitis. Twelve types of bacteria were commonly isolated with different frequencies. Anaerobes represented about 56.1 % and aerobes represented about 43.9 % of the total bacteria. Streptococcus mitis (15.2 %) was the most frequent isolated bacteria (aerobes and anaerobes) from all the cases in the 1st culture. Then it was followed by Prevotella intermedia (14.7 %), Peptostreptococcus spp. (10.5%), Eubacterium lentum (9.4 %), Lactobacillus spp. (8.9 %), Staphylococcus aureus (8.4 %), Veillonella parvula (7.9 %), Enterococcus faecalis (7.3 %), Escherichia coli (E-coli) (6.8 %), Neisseria catarrhalis (5.2 %), Proionibacterium spp. (4.7 %), Staphylococcus epidermis (1 %). The results demonstrated a highly significant reduction in the bacterial growth in the experimental groups before and after instrumentation and medication of the root canal when compared to the control. Chlorhexidine gutta percha points proved to have a significant better antibacterial effect than calcium hydroxide gutta percha points.
Keywords: Gutta percha; Periodontitis; Calcium hydroxide; Aerobic bacteria; Anaerobic bacteria
Introduction
Microorganisms are the main etiologic factors in pulpal and periapical diseases [1]. To reduce the number of microbes, many materials have been used as intracanal medicaments such as calcium hydroxide, camphorated paramonochlorophenol, iodine- potassium iodide, chlorhexidine, formocresol ,…. etc [2]. These medicaments are either used in paste or gel form and so are quite difficult to place in root canal system up to the apex and also difficult to retrieve [3-5]. These limitations focused the development of gutta percha points impregnated with calcium hydroxide and chlorhexidine, which could be placed and removed easily from the root canal [6]. Studies have been made to develop gutta percha points with antimicrobial efficacy, to function as inter-appointment intracanal dressing by incorporating calcium hydroxide or chlorhexidine [7]. Calcium hydroxide Ca(OH)2 has been extensively used as an ideal root canal dressing. The antimicrobial property of Ca(OH)2 is due to its high pH [8]. In addition, it damages the bacterial cytoplasmic membrane [8]. Chlorhexidine, a cationic bisguanide is a broad- spectrum antimicrobial agent. It has an ability to be absorbed onto dentine and onto the microorganism cell wall, causing leakage of the intracellular component [9]. None of the previous studies examined the antibacterial action of calcium hydroxide or chlorhexidine gutta percha points in vivo.
Aim of the Study
This study was designed to evaluate the microbiological analysis of teeth with chronic apical periodontitis and the effect of calcium hydroxide gutta percha points and chlorhexidine gutta percha points on the remaining bacteria after cleaning and shaping of the infected root canals in vivo.
Materials and Methods
Forty intact straight single rooted non-vital teeth were selected for the study from forty patients attending dental clinic at the college of dentistry, Suez Canal University, Egypt. Consents were taken from patients. The patients were chosen according to the following criteria; chronic periapical lesions having definite periapical radiolucency at the apex, asymptomatic, no sinus tract, negative response to palpation and percussion, no response to thermal sensitivity pulp tests, and no communication between the oral cavity and the apical lesion and enough crown structure for adequate isolation. No systemic medication of antibiotic during sample collection. Past medical history excluded those cases with systemic diseases that could interfere with tissue healing. The periapical infection was diagnosed to be of endodontic origin based on case history, clinical and radiographic examination.
Classification of the cases
The forty cases were randomly divided into three groups. Fifteen cases in each experimental group according to the material used either calcium hydroxide point’s calcium hydroxide points1 or Chlorhexidine points2. Ten cases were treated without medication and sealed by plain sterile cotton pellet to act as a control.
Sample collection
Bacterial sampling was done before and after root canal preparation as follows: the first culture was collected after access cavity opening and before instrumentation, irrigation or medication. Second culture was collected in the same visit of the first culture but after root canal instrumentation and irrigation. The third culture was collected after 7 days of medication.
First bacterial culture
All instruments (contra angle hand piece, burs, files, etc.…) were packed in sterilization pouches then sterilized in autoclave and kept closed in sterilization pouches until use. All handles and bottoms on dental units were disinfected with 5.25 % sodium hypochlorite and covered by film barrier. The patient was anesthetized and the rubber dam was applied to the treated tooth. After isolation, the tooth and the clamp were cleaned with hydrogen peroxide 30 % for 1 min. in carious teeth, caries was removed before gaining access to the pulp. An initial opening was made 1 to 22 mm in dentin with a sterile tapered fissure bur at high speed. The tooth was then swabbed by hydrogen peroxide for 10 sec., followed by application of 2% tincture of iodine for 1 min. The iodine was deactivated by 5 % sodium thiosulphate. Proceeding the penetration of the roof of the pulp chamber, a sterile surgical tungsten carbide round bur was used from inside tot outside, followed by tapers fissure to expose the entire pulp chamber. Necrotic pulp tissue remnants were removed with sterile barbed broach3 and the tooth length was determined 0.5-1 mm short of the radiographic apex. First culture for aerobic and anaerobic bacteria were collected from the canal using sterile paper points adjusted to the working length. The points were pre moistened with sterile saline to provide the pooling effect for bacterial collection [10]. Samples were collected by inserting the pre moistened paper points inside the canal until the working length and left for 2 min. Four paper points were used for culturing in each sample. The points were placed into the transporting medium (thioglycolate broth) carried in sterilized Eppendorf tubes and sent to the microbiology laboratory. Two of the paper points were transported anaerobically using anaerobic jar4 provided with gas pack generating envelope. While the other two paper points were transported under aerobic condition.
1Calcium hydroxide plus points Roeko(Germany)
2Active Points, Roeko, (Germany)
3DENTSPLY (England)
Second bacterial culture
Root canals were instrumented using modified double flared technique in the same visit of the first culture. The canals were prepared using K-flexo files5 according to their size apically. Maxillary central incisors were prepared to size 55 (master apical file). Maxillary and mandibular canines and premolars were prepared to size 45 (master apical file) as well as maxillary lateral incisors. Canals were irrigated with 10 ml of sterile saline during cleaning and shaping using sterile disposable syringe. After finishing cleaning and shaping, the second bacterial sample was collected following the same protocol of the first culture using paper point’s equivalent to the size of the master apical file. The canals were then dried by sterile paper points then medicated by the tested points (either calcium hydroxide or chlorhexidine points). The points were inserted to the working length and were sat passively so that moist air was able to circulate freely. The excess length of the points was folded in the pulp chamber to facilitate their removal. Then a sterile plain cotton pellet was applied. For the control group, a sterile plain cotton pellet was placed in the pulp chamber without any medication. A thickness of 3 mm of temporary filling (IRM)6 was packed in increments for all the groups to provide a coronal seal.
4Oxoid (England)
5DENTSPLY (Switzerland)
6DENTSPLY (Switzerland)
Third bacterial culture (7 days after the first visit)
Sterilization of the instruments, isolation and disinfection of the tooth surface was carried out as in the first visit. Temporary filling was removed sterile tapered fissure bur at high speed. Third culture was collected following the same protocol of the first and second cultures. Then all canals were irrigated using sodium hypochlorite dried and obturated with gutta percha and sealer using cold lateral compaction technique.
Laboratory part
Incubation and isolation of the microorganisms: After overnight incubation, the thioglycolate broth was sub cultured using different media and under different conditions. For isolation of aerobic bacteria, subculture was done on blood agar and MacConkey agar plates. Both media were incubated aerobically. The addition of blood provided an enriched medium needed for the growth of many microorganisms and assisted in the identification of the microorganisms that produced various types of haemolysis, in addition to its high nutritive value, blood agar was an indicator medium. Some bacteria released toxins that caused haemolysis of red cells and their colonies would be surrounded by zone of haemolysis e.g. some species of Staphylococcus and Streptococcus. MacConkey agar is a useful medium for cultivation of entero bacteria. It distinguishes between the lactose fermenting gram-negative rods (pink colonies) and non-lactose fermenting bacteria (pale colonies) [11]. For anaerobic cultivation, the inoculums were placed on pre-reduced vitamin K enriched Columbia blood agar base with hemin and anaerobic sheep blood agar plates containing kanamycin and vancomycin (selective media). Anaerobiosis was obtained by anaerobic Gas pack system in which a special jar was tightly fitting lid was used and a cold catalyst suspended from the lid consisted of alumina pellets coated with palladium and contained in gauze sachet. In this system the hydrogen was generated inside the jar by placing a special Gas pack envelope commercially prepared in which distilled water was added to the envelope immediately before placing it in the jar and it would release hydrogen and carbon dioxide. After incubation was performed, the growth checkup was done after 24 hrs. for aerobes and after 48 hrs., 4 days,7 and 14 days for anaerobes to provide time for the slow growers to produce their colonies [11].
Identification of the isolated microorganisms:
Morphological identification: Examination of Gram stained preparations determined the stating reaction of the bacteria; whether Gram +ve or Gram –ve, their morphology (Cocci and bacilli) size and arrangement [12,13].
Cultural appearance: This included the colony morphology whether it was opaque or translucent, mucoid or dry. Pigment producing bacteria formed colored colonies. If it was endopigment producer, the pigment was restricted to the colony and if it was exopigment producer m the color diffused to the surrounding medium. The type of Haemolysis on blood agar and the differentiation between the lactose fermenters that form pink colonies on MacConkey’s medium from the non-lactose fermenters [12,13].
Biochemical reactions [13]:
- Sugar carbohydrate fermentation: A method depended on the ability of bacteria with variable degrees to ferment carbohydrates with acid production with or without gas evolution.
- Indole production: The test depended on the ability of certain bacteria to break the amino acid tryptophan with the release of indole. The test bacteria were cultured in a medium, which contained tryptophan (Peptone water). Indole production was detected by Kovac’s reagent. This reacted with the indole to produce a red colored compound. Red color revealed positive insole test while no red color revealed negative indole test.
- Urease test: The test depended on the ability of certain bacteria to produce Urease enzyme, which would break down urea (by hydrolysis) to give ammonia and carbon dioxide. The test organism was cultured in a medium, which contained urea and the indicator phenol red. With the release of ammonia, the medium became alkaline as shown by a change in color of the indicator to pink red. Red color displayed positive urea test while no red color displayed negative urea test.
- Catalase test: The test used to differentiate between bacteria that produced the enzyme Catalase and non-Catalase producing bacteria, Catalase acts as a catalyst in the breakdown of hydrogen peroxide to oxygen and water. The bacteria were tested for Catalase production by bringing it into contact with hydrogen peroxide. Bubbles of oxygen are released if the bacteria are a Catalase producer. Active bubbling revealed positive Catalase test while no bubbling revealed negative Catalase test.
- Oxidase test: The test depended on the ability of certain bacteria to produce the enzyme cytochrome oxidase. Oxidase enzyme oxidized the phenylenediamine in the Oxidase reagent and gave a deep purple color. The bacteria were tested for oxidase production by bringing it into contact with a piece of filter paper soaked with a few drops of oxidase reagent. Deep purple color within 10 seconds displayed positive oxidase test while no deep purple color within 10 seconds revealed negative oxidase test.
- Coagulase test: The test depended on the ability of certain bacteria to produce the enzyme coagulase. Coagulase enzyme caused plasma to clot by converting fibrinogen to fibrin. The bacteria were tested for coagulase production by bringing it into contact with human or rabbit plasma on a slide. Clumping within 10 seconds revealed positive coagulase test while no clumping within 10 seconds revealed negative coagulase test.
- API system7: A method that could identify a wide variety of microorganisms. The system comprised of strips that generally contained 20 miniature biochemical tests covering almost most of the bacterial groups as well as different species. This system was specially used for anaerobic identification.
7BioMeieux (France)
Counting of microorganisms: A calibrated loop was used to carry a calibrated amount from the transport medium (100 Micron), then diluted in different dilutions and cultured on different plates. The former colonies were counted from the plates and their count was multiplied by the dilution to give the total bacterial count of the sample.
Data management: The data were collected, tabulated and subjected to statistical analysis. Chi-square was used to compare between proportions of the number of isolated bacteria. Paired samples t- test was utilized to compare the mean values of colony counts within the same group in the 1st, 2nd and 3rd cultures. T- Test for independent variables was utilized to compare the mean values of colony count among the different groups.
Results
Identification and description of bacteria in infected root canals with periapical lesions
All cases revealed growth of both aerobic and anaerobic bacteria. The most common isolate bacteria (aerobes and anaerobes) and their frequency from all cases of the different groups in the 1st culture were presented in (Figure 1). Twelve types of bacteria were commonly isolated with different frequencies. Anaerobes represented about 56.1 % and aerobes represented about 43.9 % of the total bacteria. Streptococcus mitis (15.2 %) was the most frequent isolated bacteria (aerobes and anaerobes)from all the cases in the 1st culture. Then it was followed by Prevotellaintermedia (14.7%), Peptostreptococcusspp. (10.5%), Eubacteriumlentum (9.4%), Lactobacillusspp. (8.9%), Staphylococcusaureus (8.4%), Veillonellaparvula (7.9 %), Enterococcusfaecalis (7.3 %), Escherichia coli (E-coli) (6.8%), Neisseriacatarrhalis(5.2%), Proionibacterium spp. (4.7%), Staphylococcusepidermis (1%).

Figure 1: Percentage of common bacteria isolated from all cases in the 1st culture.
The effect of instrumentation and irrigation on the present bacteria in infected root canals: All cases revealed positive cultures of both aerobic and anaerobic bacteria after mechanical instrumentation and irrigation. Figure 2 represented the percentage of common bacteria (aerobes and anaerobes) remained in the root canals after instrumentation and irrigation. Prevotella intermedia (19.8 %) was the most frequent isolated bacteria (aerobes and anaerobes) from all the case in the 2nd culture. Then it was followed by Streptococcus mitis (13.5%), Peptostreptococcus spp. (13.5%), Eubacteriumlentum (13.4%), Enterococcus faecalis (11.1),and Lactobacillus spp. (11.1), Veillonella parvula (7.9%), and Proionibacterium spp. (4 %)
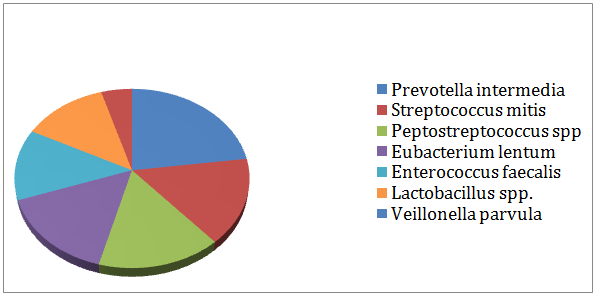
Figure 2: Percentage of common bacteria isolated from all cases in the 2nd culture.
Table 1 revealed the total number of bacteria isolated from the 1st and 2nd cultures in all cases. In-group I, the number of isolated bacteria was 71 in the 1st culture, 50 in the 2nd culture. In-group II, the number of isolated bacteria was 72 in the 1st culture, 45 in the 2nd culture. In the control group, the number of isolated bacteria was 48 in the 1st culture, 31 in the 2nd culture. No significant difference was found between groups regarding the number of bacteria isolated from the 1st and 2nd cultures. In spite of evidence of positive bacterial growth of all 2nd cultures after instrumentation, the total bacteria count revealed a significant decline from the 1st to the 2nd cultures. As shown in (Table 2), all groups showed a significant difference between the mean colony count ( CFU x 103 /0.1 of inoculums) of both aerobes in the 1st culture ( 162.7 ± 133.5, 177.3 ± 174.1, 169 ± 100.3) and anaerobes (193.3 ± 77.1, 202 ± 126.4, 214.5 ± 99.2) and that of the 2nd culture (42.2 ± 35.8, 50.7 ± 59.2, 50 ± 40) for aerobes (55 ± 43.1, 78.9 ± 59.2, 83 ± 29.8) for anaerobes for anaerobes for group I, II and the control group respectively. Regarding the comparison between the mean colony counts of all groups, there was no significant difference in mean colony count between the three groups in the 1st and the 2nd cultures for both aerobes and anaerobes (Tables 3-5).
No. of Isolated Bacteria |
|
1st Culture |
2nd Culture |
X2 |
P |
Group I ( N= 15) |
71 |
50 |
0.09 |
0.769 |
Control Group ( N= 10) |
48 |
31 |
Group II ( N= 15) |
72 |
45 |
0.01 |
0.913 |
Control Group ( N= 10) |
48 |
31 |
Group I ( N= 15) |
71 |
50 |
0.2 |
0.652 |
Group II ( N= 15) |
72 |
45 |
Table 1: Comparison between the numbers of bacteria isolated from all cases in the 1st and 2nd cultures.
Mean Colony Count |
( CFU x 103 /0.1 of Inoculums) |
|
1st Culture |
2nd Culture |
Mean Difference |
P |
Group I ( N= 15) |
|
|
|
|
Aerobic Bacteria |
162.7 ± 133.5 |
42.2 ± 35.8 |
120.5 |
0.001 |
Anaerobic Bacteria |
193.3 ± 77.1 |
55.0 ± 43.1 |
138.3 |
0 |
Total |
356.0 ± 160.9 |
97.2± 60.2 |
258.8 |
0 |
Group II (N= 15) |
|
|
|
|
Aerobic Bacteria |
177.3±174.1 |
50.7± 59.2 |
126.7 |
0.001 |
Anaerobic Bacteria |
202.0 ±126.4 |
78.9± 59.2 |
123.1 |
0 |
Total |
379.3±234.3 |
129.5 ± 84.1 |
249.8 |
0 |
Control Group I ( N= 10) |
|
|
|
0 |
Aerobic Bacteria |
169.0± 100.3 |
50.0± 40.0 |
119 |
0.001 |
Anaerobic Bacteria |
214.5± 99.2 |
83.0 ± 29.8 |
131.5 |
0 |
Total |
383.5± 188.2 |
133.0± 60.9 |
250.5 |
|
Table 2: Comparison between the mean colony counts of bacteria isolated from all cases in the 1st and 2nd cultures.
Mean Colony Count |
( CFU x 103 /0.1 of Inoculums) |
|
Group I |
Control |
Mean Difference |
P |
( N=15) |
(N=10) |
1st Culture |
|
|
|
|
Aerobic Bacteria |
162.7±133.5 |
169.0±100.3 |
6.3 |
0.9 |
Anaerobic Bacteria |
193.3±77.1 |
214.5±99.2 |
21.2 |
0.554 |
Total |
356.0±160.9 |
383.5±188.2 |
27.5 |
0.705 |
2nd Culture |
|
|
|
|
Aerobic Bacteria |
42.2±35.8 |
50±40.0 |
7.8 |
0.615 |
Anaerobic Bacteria |
55.0±43.1 |
83.0±29.8 |
28 |
0.087 |
Total |
97.2±60.2 |
133.0±60.9 |
35.8 |
0.161 |
3rd Culture |
|
|
|
|
Aerobic Bacteria |
7.3±16.1 |
49.0±37.3 |
41.7 |
0.006 |
Anaerobic Bacteria |
18.7±24.1 |
91.5±26.5 |
72.8 |
0 |
Total |
25.9±29.0 |
140.5±55.9 |
114.6 |
0 |
Table 3: Comparison between the mean colony counts of bacteria isolated from cases treated by calcium hydroxide points (Group I) and control group in the 1st, 2nd and 3rd cultures.
Mean Colony Count |
(CFU x 103 /0.1 of Inoculums) |
|
Group II |
Control |
Mean Difference |
P |
( N=15) |
(N=10) |
1st Culture |
|
|
|
|
Aerobic Bacteria |
177.3±174.1 |
169.0±100.3 |
8.3 |
0.881 |
Anaerobic Bacteria |
202.0±126.4 |
214.5±99.2 |
12.5 |
0.795 |
Total |
379.3±234.3 |
383.5±188.2 |
4.2 |
0.961 |
2nd Culture |
|
|
|
|
Aerobic Bacteria |
50.7±59.2 |
50±40.0 |
21.4 |
0.667 |
Anaerobic Bacteria |
78.9±59.2 |
83.0±29.8 |
4.1 |
0.841 |
Total |
129.5±84.1 |
133.0±60.9 |
3.5 |
0.912 |
3rd Culture |
|
|
|
|
Aerobic Bacteria |
0.0±16.1 |
49.0±37.3 |
49 |
0.002 |
Anaerobic Bacteria |
0.5±1.5 |
91.5±26.5 |
91 |
0 |
Total |
0.5±1.5 |
140.5±55.9 |
140 |
0 |
Table 4: Comparison between the mean colony counts of bacteria isolated from cases treated by chlorhexidine points (Group II) and control group in the 1st, 2nd and 3rd cultures.
Mean Colony Count |
( CFU x 103/0.1 of Inoculums) |
|
Group I |
Group II |
Mean Difference |
P |
( N=15) |
( N=15) |
1st Culture |
|
|
|
|
Aerobic Bacteria |
162.7 ± 133.5 |
177.3 ± 174.1 |
14.7 |
0.881 |
Anaerobic Bacteria |
193.3 ± 77.1 |
202.0± 126.4 |
8.7 |
0.795 |
Total |
356.0 ± 160.9 |
379.3 ± 234.3 |
23.3 |
0.961 |
2nd Culture |
|
|
|
|
Aerobic Bacteria |
42.2± 35.8 |
50.7± 59.2 |
8.5 |
0.667 |
Anaerobic Bacteria |
55.0 ± 43.1 |
78.9 ± 59.2 |
23.9 |
0.841 |
Total |
97.2± 60.2 |
129.5± 84.1 |
32.3 |
0.912 |
3rd Culture |
|
|
|
|
Aerobic Bacteria |
7.3± 16.1 |
0.0± 16.1 |
7.3 |
0.002 |
Anaerobic Bacteria |
18.7± 24.1 |
0.5± 1.5 |
18.1 |
0 |
Total |
25.9± 29.0 |
0.5± 1.5 |
25.4 |
0 |
Table 5: Comparison between the mean colony counts of bacteria isolated from cases treated by calcium hydroxide points (Group I) and cases treated by chlorhexidine points (Group II) in the 1st, 2nd and 3rd cultures.
The effect of medication on the bacterial growth
In-group I, (Figure 3) displayed the remaining aerobic and anaerobic bacteria in the 3rd culture. 79% of the cases produced negative cultures after medication with calcium hydroxide gutta percha points for 7 days. Enterococcus faecalis (6%) and Eubacteriumlentum (6%) were the most resistant bacteria followed by Prevotella intermedia (4%) Peptostreptococcus spp. (3%), Streptococcus mitis (1%) and Veillonellaparvula (1%). In-group II, (Figure 4) showed the remaining aerobic and anaerobic bacteria from the 3rd culture. 98% of the cases produced negative cultures after the medication with the chlorhexidine gutta percha points for 7days. Eubacteriumlentum was isolated from a single case and so were Prevotella intermedia.
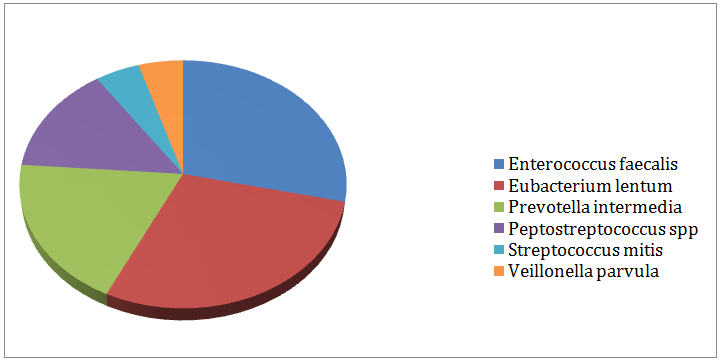
Figure 3: Remaining bacteria after medication with calcium hydroxide (Group I).
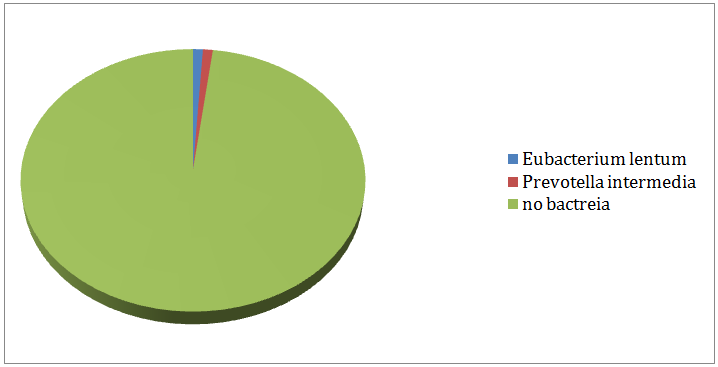
Figure 4: Remaining bacteria after medication with chlorhexidine (Group II).
Effect on the number of isolated bacteria: Statistical analysis of isolated bacteria using Chi Square test (Table 6) revealed a highly significant difference between the 2nd and 3rd cultures of the experimental groups only (group I and II). There was a highly significant difference between group I and II. As shown in (Figure 5) in-group I, the number of isolated bacteria was 50 in the 2nd culture and 15 in the 3rd culture. In-group II the number of the isolated bacteria were 45 in the 2nd culture and 2 in the 3rd culture. In the control group, 31 isolates of bacteria in both cultures.
No. of Isolated Bacteria |
1st Culture 2nd Culture X2 P |
Group I ( N= 15) |
50 |
15 |
9.96 |
0.002 |
Control Group ( N= 10) |
31 |
31 |
Group II ( N= 15) |
45 |
2 |
26.5 |
0 |
Control Group ( N= 10) |
31 |
31 |
Group I ( N= 15) |
50 |
15 |
7.51 |
0.006 |
Group II ( N= 15) |
45 |
Table 6: Comparison between the number of bacteria isolated in the 2nd and 3rd cultures from cases treated by calcium hydroxide points (Group I), Chlorhexidine points (Group II) and control group.
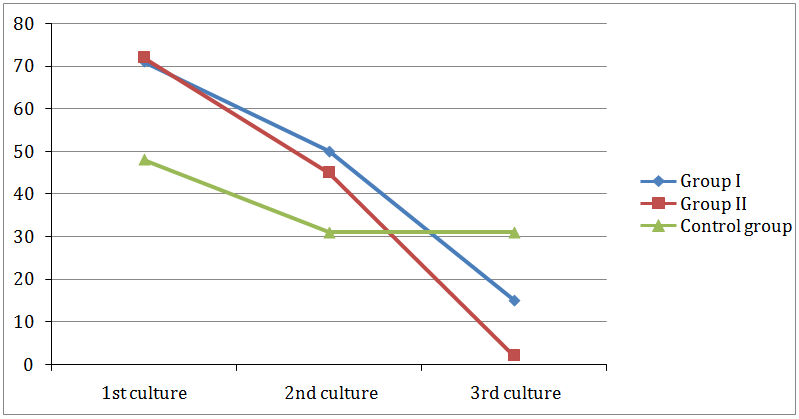
Figure 5: Comparison between numbers of bacteria isolated from 1st, 2nd and 3rd cultures in group I, group II and control group.
Effect on the mean colony count: Both experimental groups (group I and II) showed a highly significant decrease in the mean colony count between the mean colony count of the control group in the 2nd and 3rd cultures. Group I revealed mean colony count (CFU x 103 / 0.1 of inoculum ) of 97.2 ± 60.2, 42.2+ 35.8, and 55 ± 43.1 in the 2nd culture and 25.9 ±29, 7.3 ± 16.1 and 18.7 ± 24.1 for the 3rd culture for total, aerobic and anaerobic bacteria respectively. Group II revealed mean colony count of 129.5± 84.1, 50.7+ 59.2 and 78.9± 59.2 in the 2nd culture and 0.5 ±1.5, 0 and 0.5 ± 1.5 for the 3rd culture for total, aerobic and anaerobic bacteria respectively. Table 7, Figure 5).
Mean Colony Count |
(CFU x 103 /0.1 of Inoculums) |
2nd Culture 3rd Culture Mean Difference P |
Group I ( N= 15) |
|
|
|
|
Aerobic Bacteria |
42.2 ± 35.8 |
7.3 ± 16.1 |
34.9 |
0 |
Anaerobic Bacteria |
55.0 ± 43.1 |
18.7 ± 24.1 |
36.3 |
0 |
Total |
97.2± 60.2 |
25.9± 29.0 |
71.3 |
0 |
Group II ( N= 15) |
|
|
|
|
Aerobic Bacteria |
50.7± 59.2 |
0 |
50.7 |
0 |
Anaerobic Bacteria |
78.9± 59.2 |
0.5± 1.5 |
78.3 |
0 |
Total |
129.5 ± 84.1 |
0.5± 1.5 |
129 |
0 |
Control Group I ( N= 10) |
|
|
|
|
Aerobic Bacteria |
50.0± 40.0 |
49.0± 37.3 |
1 |
0.798 |
Anaerobic Bacteria |
83.0 ± 29.8 |
91.5 ± 26.5 |
8.5 |
0.212 |
Total |
133.0± 60.9 |
140.5± 55.9 |
7.5 |
0.315 |
Table 7: Comparison between the mean colony count of bacteria isolated in the 2nd and the 3rd cultures from cases treated by calcium hydroxide points (Group I), Chlorhexidine points (Group II) and control group.
Comparison between the mean colony counts of bacteria in the 3rd culture of all groups: As represented in (Table 3) group I revealed a highly significant difference in the mean colony count of the total bacteria 25.9 ± 29, for aerobes 7.3 ± 16.1 and anaerobes 18.7 ± 24.1 when compared with that of the control group 140.5 ± 55.9 for the total, for aerobes 49 ± 37.3 and 91.5 ± 26.5 for anaerobes. As shown in (Table 4), group II revealed a highly significant difference in the mean colony count of the total bacteria 0.5 ±1.5, for aerobes 0 and anaerobes 0.5± 1.5 when compared with that of the control group 140.5+ 55.9 for the total, for aerobes 49 + 37.3 and 91.5 + 26.5 for anaerobes. As displayed in (Table 5), group II showed a significant difference in the mean colony count of bacteria (0.5 ± 1.5, 0, 0.5 ± 1.5) than that of group I (25.9 ± 29, 7.3 ± 16.1, 18.7 + 24.1) for total, aerobic and anaerobic bacteria respectively. No difference was found between group I and II for the mean colony count of aerobes in the 3rd culture (Figure 6).
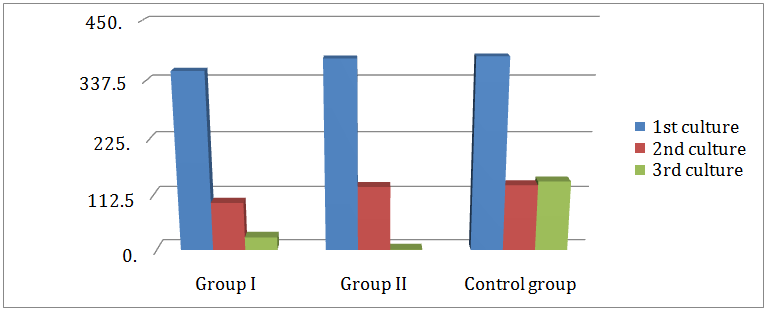
Figure 6: Comparison between mean colony count of aerobic and anaerobic bacteria isolated form 1st, 2nd and 3rd cultures in group I, group II and control group.
Discussion
Successful endodontic treatment depends on elimination of bacteria and their byproducts from the pulp space and the removal of the substrate on which they depend on. Biomechanical instrumentation and irrigation are helpful tools in cleaning the root canal system. Unfortunately, bacteria may remain viable even after thorough chemo mechanical preparation jeopardizing the outcome of the root canal treatment [1]. Intracanal medication has been advocated to destroy the remaining bacteria and maintain the ultimate cleanliness of the root canal. The microbial composition of infected root canals is an important factor in the selection of intra canal dressing [2]. The antimicrobial action of this dressing must cover the different types of microorganisms killing them or at least reduce their number to favor repair. In order to produce a clinical reliable data concerning the antibacterial effect, an in vivo study was conducted. Non-vital teeth with chronic periapical periodontitis were selected to be sure of the existence of bacteria. In order to evaluate the antimicrobial action of the tested materials, 3 cultures were taken before, after instrumentation and 7 days after medication to maximize the effect of the medicaments. A transport medium of thioglycolate was used to preserve and support the growth of both aerobic and anaerobic bacteria during transportation [12,13]. In this study, Streptococcus mitis, Prevotellaintermedia, Peptostreptococcus spp, Eubacteriumlentum, Lactobacillus spp. were the most dominate isolated bacteria from the infected root canals at the access opening before instrumentation. This finding was in accordance with that obtained by Sundqvist [14]. Likewise, the percentage of the anaerobic bacteria was found to be greater than the aerobic bacteria, which coincided with the results reported by Baumgartner and Falker [15]. A significant bacterial reduction following instrumentation and irrigation was observed in the present study, which is in agreement with, that obtained by Dalton et al. [16]. The cause might be due to the cumulative effects of both mechanical removals by instrumentation using modified double flared technique and flushing mode of saline irrigant. On the other hand, some bacteria remained after instrumentation and irrigation, which might be attributed to two reasons. One of them was that saline has no antibacterial action but worked only on its flushing effect. The second reason was that bacterial cells might be closed within anatomical in accessible areas to the saline irrigant. In this study, calcium hydroxide and chlorhexidine (Ca(OH)2, CHX) incorporated within gutta percha points were evaluated as intra canal medicaments. These points were introduced into the market to permit slow release of the ingredients (Ca(OH)2, CHX) over longer periods in addition, to overcome the problems of application and removal associated with the use of the other forms of these materials. When Ca(OH)2 points were used, 79 % of the cases showed negative cultures. The great antibacterial effect of the Ca(OH)2 points was in agreement with that obtained by other authors [17-19]. Te mechanism of action of Ca(OH)2 as antibacterial agent may be due to the liberation of hydroxyl ions (OH)- and inactivation of enzymes of the cytoplasmic membrane of bacteria which chemically altered the organic components and nutrient transport causing toxic effects on the cells [20]. Safavi and Nichols [21] demonstrated that Ca(OH)2 had the capacity too hydrolyze the lipid moiety of bacterial LPS resulting in the release of free hydroxyl fatty acids promoting lipopolysaccharides degradation. The presence of sodium chloride as a surfactant within the points might have the influence of the release of (OH) - that increased the pH level, which may be lethal to bacteria. The release of (OH) - might be initiated by the fluid flow into the spaces between the points and the canal wall from the dentinal tubules and the apical area. Beside the main ingredient of the tested materials Ca(OH)2, zinc oxide might play additional role in the antibacterial process [21]. On the contrary, some authors recognized that Ca(OH)2 points did not produce sterile cultures although bacterial reduction [22-25]. These studies are partially in agreement with the present results where 21 % of cases treated by Ca(OH)2 points showed positive cultures which might be due to many factors. Firstly, to be effective against bacteria located inside the dentinal tubules, the (OH)_ from Ca(OH)2 points should diffuse into dentin at sufficient concentration exceeding the dentin buffer ability which might not occur in all cases. Greater release of the ions was facilitated in the beginning, but ingress of tissue fluid from the dentinal tubules or the exudate or pus from the periapical area might challenge ionic diffusion that might adversely affect high alkalinity of the material [26]. Secondly, the gutta percha points probably might bind the (OH)_ blocking their release at the site of application [27]. Thirdly, the Ca(OH)2 points inherently might not have the ability to alkalinize dentin [28,29] or unable to sustain the alkalinity by time [30]. This decline might be as a result of the neutralizing effect of carbon dioxide presented in body fluids or originated from bacterial metabolism in canals or dentinal tubules [31]. One major factor could alter the pH is the degree of calcification of dentin, which might affect the permeability of (OH) _ and thus affects the pH [20]. Fourthly, it might be possible that bacteria presented in the dentinal tubules escaped the direct action of Ca(OH)2 . Finally, some microorganisms could survive in high pH such as Enterococcus faecalis and Prevotella intermedia, which considered as the most common resistant bacteria to Ca(OH)2 many researchers assured this result [1]. They found that some bacteria might be located as small colonies in which cells in the center could be protected by cells lying in the periphery. Moreover, AlNazhan et al. demonstrated a weak anti-bacterial action of Ca(OH)2 points that permitted bacteria survival in their studies [32].
Chlorhexidine points displayed a strong antimicrobial action against all bacteria with 98% incidence of negative cultures. This potent antibacterial effect of CHX might be explained by the fact that CHX cationic molecules might bind to the negatively charged bacterial cell wall, thereby altering the cell osmotic equilibrium causing disruption of bacterial cytoplasmic membrane [33]. In addition to its bacteriostatic effect when it was located inside the root canal at lower concentration [21], zinc oxide might also add to the antibacterial action of these points. Previously, CHX was used in the form of solution or gel, which was not suitable to sufficiently remain within the canals for longer periods, so attempts had been tried to dispense CHX from a controlled or sustained release devise. In these devises, CHX was contained in a polymer sheath that when placed in a liquid environment gradually dissolved and released CHX. Those devises were the predecessors for the CHX points, where some of them presented satisfied results while others failed [34,35]. In the present study CHX, points displayed a significant better antibacterial effect than Ca(OH)2 points. This finding was compatible with that obtained by others [22-24] who reported that CHX showed complete elimination of bacteria especially Enterococcus faecalis. Opposite to our finding, Barthel et al. [36] reported only bacterial reduction after application of CHX points. Different methodologies might be the cause of difference in results, beside what they reported about the rigidity for the points that might restrict the release of CHX. Still, their results resembled the present study in that CHX points produced better action than that of Ca(OH)2 [36]. Two out of fifteen cases treated by CHX revealed positive cultures. That might be because of tissue component and bacterial effect that might neutralize the medicament. Bacteria might alter their pattern of gene expression after changes in the environmental conditions, which allowed them to survive in unfavorable conditions [14].
In the control group, most of the cases revealed an increase in the bacterial growth in the 3rd culture than 2nd culture. This might be due to the ideal conditions existing for bacterial multiplication on the remaining bacterial substrate within the root canal system. However, few cases showed reduction in bacterial growth with the 2nd culture although the reduction was not significant. There might be impairment in the synergism between bacteria with each other, as some species could not survive in the root canal by itself without its companion that was might be flushed by Sundqvist [14].
Conclusion
- All treated infected non-vital teeth with periapical lesions in this study displayed positive bacteria cultures of both aerobic and anaerobic bacteria with predominance of anaerobes.
- Chlorhexidiene gutta percha points when used as intracanal medicament revealed a highly significant antibacterial effect against both aerobic and anaerobic bacteria remained after mechanical instrumentation of the infected root canals.
- Calcium hydroxide gutta percha points when used as intra canal dressing showed significant antibacterial effect against most of the aerobic bacteria while most of the anaerobic bacteria revealed resistance.
References
- Siren EK, Haapasalo MP, Waltimo TM, Orstavik D (2004) In vitro antibacterial effect of calcium hydroxide combined with chlorhexidine or iodine potassium iodide on Enterococcus faecalis. Eur J Oral Sci 112(4): 326-331.
- Almyroudi A, Mackenzie D, McHugh S, Saunders WP (2002) The effectiveness of various disinfectants used as endodontic intra canal medicaments: an in vitro study. J Endod 28(3): 163-167.
- Fava LR, Saunders WP (1999) Calcium hydroxide pastes: classification and clinical indications. Int Endod J 32(4): 257-282.
- Lambrianidis T, Kosti E, Boutsioukis C, Mazinis M (2006) Removal efficacy of various calcium hydroxide/chlorhexidine medicaments from the root canal. Int Endod J 39(1): 55-61.
- Menezes MM, Valera MC, Jorge AO, Koga-Ito CY, Camargo CH, et al. (2004) In vitro evaluation of the effectiveness of irrigants and intracanal medicaments on microorganisms. Int Endod J 37(5): 311-319.
- Ebert J, Roggendorf MJ, Frank K, Petschelt A (2008) Antimicrobial activity of various ‘active’ gutta percha points against Enterococcus faecalis in simulated root canals. Int Endo J 41(3): 249-257.
- Vijay R, Makam S, Shashikala K (2010) Evaluation of antimicrobial efficacy of chlorhexidine gutta percha and calcium hydroxide gutta percha against Enterococcus faecalis - an in vitro study. Streamdent 1(3): 209- 213.
- Vianna ME, Gomes BP, Sena NT, Zaia AA, Ferraz CC, et al. (2005) In vitro evaluation of the susceptibility of endodontic pathogens to calcium hydroxide combined with different vehicles. Braz Dent J 16(3): 175-180.
- Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, et al. (2003) Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J 36(4): 267-275.
- Marshell FJ, Savoic FL (1967) Efficiency of endodontic culturing procedures using wet and dry paper points. Oral Surg Oral Med Oral Pathol 23(6): 806-810.
- Mackie, McCartney (1996) Practical Medical Microbiology. (14th edn), Churchill living stone, Edinburgh, UK, pp. 107-108.
- Monica C (1984) Medical laboratory manual for topical countries. Volume II, Cambridge University Press, UK, 53: 175-178.
- Monica C (1999) District laboratory practice in tropical countries. Volume II, Cambridge University Press, UK, pp. 60, 83,126,128.
- Sundqvist G (1992) Ecology of the root canal flora. J Endod 18(9): 427-430.
- Baumgartner JC, Falker WA (1991) Bacteria in the apical 5-mm of infected root canals. J Endod 17(8): 380-383.
- Dalton BC, Orstavik D, Phillips C, Pettiette M, Trope M (1998) Bacterial reduction with nickel titanium rotary instrumentation. J Endod 24(11): 763-767.
- Ebert J, Roggendorf MJ, Frank K, Petschelt A (2008) Antimicrobial activity of various ‘active’gutta-percha points against Enterococcus faecalis in simulated root canals. Int Endod J 41(3): 249-257.
- Podbielski A, Boeckh C, Haller B (2000) Growth inhibitory activity of gutta-percha points containing root canal medications on common endodontic bacterial pathogens as determined by an optimized quantitative in vitro assay. J Endod 26(7): 398-403.
- Singh H, Kapoor P (2014) A Comparative evaluation of Antibacterial Efficacy of ‘Activ Points’And ‘Combi Points’as Intra-Canal Medicaments against Enterococcus faecalis: An Ex Vivo Study. Oral Health Dent Manag 13(3): 610-613.
- Estrela C, Pimenta FC, Ito IY, Bammann LL (1999) Antimicrobial evaluation of calcium hydroxide in infected dentinal tubules. J Endod 25(6): 416-418.
- Safavi KE, Nichols FC (1993) Effect of calcium hydroxide on bacterial lipopolysaccharide. J Endod 19(2): 76-78.
- Tanomaru JM, Pappen FG, Tanomaru Filho M, Spolidorio DM, Ito IY (2007) In vitro antimicrobial activity of different gutta-percha points and calcium hydroxide pastes. Braz Oral Res 21(1): 35-39.
- Naik B, Shetty S, Yel M (2013) Antimicrobial activity of gutta-percha points containing root canal medications against E. faecalis and Candida albicans in simulated root canals- An in vitro study. Endodontology 25(2): 8-18.
- Alagarsamy A, Ebenezar AVR, Srinivasan MR , Mohan AG , Kumar S (2013) Effectiveness of calcium hydroxide plus points and chlorhexidine activ points against Enterococcus faecalis by agar diffusion test: An in vitro study. JRD 1(1): 18-21.
- Holland R, Murata SS, Dezan E, Garlipp O (1996) Apical leakage after root canal filling with an experimental calcium hydroxide gutta-percha point. J Endod 22(2): 71-73.
- Larsen MJ, Horsted-Bindslev P (2000) A laboratory study evaluating the release of hydroxyl ions from various calcium hydroxide products in narrow root canal like tubes. Int Endod J 33(3): 238-242.
- Economides N, Koulaouzidou EA, Beltes P, Kortsaris AH (1999) In vitro release of hydroxyl ions from calcium hydroxide gutta percha points. J Endod 25(7): 481-482.
- Ardeshna SM, Qualtrough AJ, Worthington HV (2002) An in vitro comparison of pH changes in root dentine following canal dressing with calcium hydroxide points and a conventional calcium hydroxide paste. Int Endod J 35(3): 239-244.
- Azabal-Arroyo M, Menasalvas-Ruiz G, Martin-Alonso J, Arroquia JJ, Vega-del Barrio JM (2002) Loss of hydroxyl ions from gutta percha points with calcium hydroxide in their composition: an in vivostudy. J Endod 28(10): 697-698.
- Schafer E, Albehiassi A (2000) pH changes in root dentin after root canal dressing with gutta percha points containing calcium hydroxide. J Endod 26(11): 665-667.
- Fuss Z, Rafaeloff R, Tagger M, Szajkis S (1996) Intracanal pH changes of calcium hydroxide pastes exposed to carbon dioxide in vitro. J Endod 22(7): 362-364.
- Al-Nazhan S (2002) Antimicrobial activity of extracts of calcium hydroxide points. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93(5): 593-595.
- Greenstein G, Berman C, Jaffin R (1986) Chlorhexidine an adjunct to periodontal therapy. J. Periodontal 57(6): 370-377.
- Heling I, Sommer M, Steinberg D, Friedman M, Sela MN (1992) Microbiological evaluation of the efficacy of Chlorhexidine in a sustained-release device for dentin sterilization. Int Endod J 25(1): 15-19.
- Lenet BJ, Komorowski R, Wu XY, Huang J, Grad H, et al. (2000) Antimicrobial substantivity of Bovine root dentin exposed to different chlorhexidine delivery vehicles. J Endod 26(11): 652-655.
- Barthel CR, Zimmer S, Zilliges S, Schiller R, Gobel UB, et al. (2002) In situ antimicrobial effectiveness of chlorhexidine and calcium hydroxide: gel and paste versus gutta percha points. J Endod 28(6): 427-430.

©2015 Sharaan. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.




