Journal of
eISSN: 2373-4345


Research Article Volume 12 Issue 1
1Departamento de Periodoncia, Universidad del Desarrollo, Chile
2Center for Advanced Prosthodontics and Implant Dentistry, University of Concepcion, Chile
3Laboratorio de Nanociencias y Nanotecnología (FCFM), (CIIDIT), Universidad Autónoma de Nuevo León, México
4Department of Restorative Dentistry, Endodontic Discipline, Facultad de Odontología, Universidad de Concepción, Chile
5Unidad de Desarrollo Tecnológico, Chile
6Interdisciplinary Group of Applied Nanotechnology (GINA), Hybrid Materials Laboratory (HML), Department of Materials Engineering (DIMAT), Faculty of Engineering, University of Concepcion, Chile
Correspondence: Andrés Gómez Morales, Departamento de Periodoncia, Universidad del Desarrollo, Chile. Ainavillo 456 Concepción, Chile
Received: January 18, 2021 | Published: February 4, 2021
Citation: Gómez A, Jofre J, Perez-Tijerina E, et al. Effect of titanium coated with 3 different types of copper nanoparticles in the oral biofilm formation. J Dent Health Oral Disord Ther. 2021;12(1):1-6. DOI: 10.15406/jdhodt.2021.12.00541
The antimicrobial activity of copper nanoparticles (CuNps) has been studied against different pathogenic microorganisms, however to our knowledge, no studies have been reported about their activity against periodontal bacteria in a biofilm. Therefore, in order to bridge this information gap, this study aims to observe and count the formation of oral biofilm on titanium alloys coated with different types of CuNps. Three different methods were used to synthetize and then apply a coating of CuNps on dental implant healing caps, by then, their antibacterial properties were investigated using an in vitro oral biofilm by plate count method and confocal laser microscopy.
The result of the counts, showed that the lower microbial load is observed in the caps coated with CuNps obtained by copper electroplating, it can be concluded, within the limitations of this study, that CuNps obtained by copper electroplating showed greater bactericidal effect that PVD methods, especially in periodontal pathogenic bacteria like P.gingivalis, and P. intermedia. More studies are necessary for corroborate this observation and better understand the reason why only CuNps obtained by certain methods were more bactericidal tan others and the reason why only some bacteria were affected. The antimicrobial properties exhibited by CuNps could be useful for develop anti-infective biomaterials become a strategy to control dental biofilm.
Keywords:copper nanoparticles; titanium alloys; antimicrobial activity; biofilm.
Titanium alloys have been widely used as bone implant materials in applications such as bone screws, as well as plate and dental implants, due to their good biocompatibility, corrosion resistance and mechanical properties.1,2 However, bacterial infection after implant placement is still a significant complication, as it might lead to implantation failure.1,3
Over the last years4-6 one alternative for avoiding bacterial infection is the use of “nanoparticles”, especially those with a size between 1 and 100 nm size that behave as a whole unit with respect to transport and properties, these particles with greater surface area than conventional materials are currently considered as potential antimicrobial agents5,6 and their use as inhibition agents of microbial growth, has emerged due to the development of antibiotic resistance.5,7,8 Among these nanoparticles, Copper nanoparticles (CuNps) have been used more in medical applications by their less toxicity and antibacterial activity.4,9-12
The bactericidal effect of CuNps has been attributed to their small size and high surface to volume ratio, which allow them to interact closely with microbial membranes and it is not merely due to the release of metal ions in solution.4,9,10,12 The antibacterial and antifungal effect of CuNps was reported by Yoon et al.12,13 and Cioffi et al.12,14 Nowadays these biofilm control properties are receiving increasing attention for a potential application in controlling infections within the oral cavity.15,16
Microbiota of dental implants and that of teeth, in healthy and periodontitis conditions are similar15 and support the concept that periodontal pathogens (A. actinomycetemcomitans, A. naeslundii, F. nucleatum, P. gingivalis, and P. intermedia, among others) may be involved in periimplant infections. These data strengthen the hypothesis that periodontally compromised patient have an increased susceptibility to Periimplantitis.15,17 The number of bacteria species in oral biofilm is currently undetermined and becomes increasingly higher as advances in molecular techniques allow for more specific identification profiles. Research on dental plaque is, hence, difficult, because it involves biofilms containing more than 700 different bacterial species, which can vary from one individual to another or within specific sites of the oral environment, and this microbial composition reflects complex processes within the oral cavity which are influenced by a multitude of endogenous and exogenous factors, besides the large number of interactions that take place between bacteria.18 All these conditions make it preferable to study these bacteria and biofilms in controlled environments labs where mouth conditions are simulated.
Periimplantitis is described as inflammatory process, affecting tissues around an osseointegrated implant in function, resulting in loss of supporting bone.19 These are usually associated with an increase in the level of pathogenic bacteria from the orange and red complexes. P.gingivalis and Tannerella forsythia are the most common and abundant red complex species, whereas Prevotella nigrescens, Prevotella oris and F. nucleatum are frequently isolated periodontal pathogens from the orange complex. However, the microflora of infected periimplant sites is found to be much more diverse than in periodontitis.19,20
The antimicrobial activity of CuNps has been studied against different pathogenic microorganisms1,2,4,9,16,17,21 however, to our Knowledge, no studies have been reported about their activity against periodontal bacteria in a biofilm. Biofilms are a complex microbial community and are known to be a significant problem for dental implants, as biofilm formation protects pathogenic bacteria against antibiotics and is one of the main causes of development of periimplantitis.15
Therefore, in order to bridge this information gap, this study aims to observe the formation of oral biofilm, on titanium alloys covered with different copper nanoparticles coatings.
Preparation of Caps with CuNPs Coatings
In the present work, dental implant healing caps of titanium aluminum vanadium alloy (Ti-6AI-4V), of the company Alphabio Tec®, of 5mm diameters and 3mm long. Chemical compositions of materials were shown in Table 1. Microstructure was Widmannstetter structure; the hardness of materials was 29.5 HRC.
Fe |
C |
N |
H |
O |
Al |
V |
Ti |
0.25 |
0.09 |
0.05 |
0.013 |
0.02 |
6.1 |
4.1 |
Balance |
Table 1 Chemical composition of the tested sample (in wt.%)
Prior to deposition, the specimens were chemically cleaned in a heated (60 °C) ultrasonic bath using a three-stage process with ten minutes allotted per stage. In the first stage, the specimens were de-greased in trichloroethylene, the second stage involved using acetone and the third stage used methanol.
Tree different types of methods were used to make the copper deposits on the caps, which were: Group 1) Copper electroplating; Group 2) Physical Vapor Deposition (PVD); Group 3) Direct Current (DC) Sputtering and PVD. The experimental design was made up of 24 caps. 6 caps for each method described above for a total of 18 and 6 for the uncoated control sample o Group 4.
For the first method (Copper electroplating) a current density of 2.5 A/dm2 to obtain about a 10 μm-thick Cu plating on the six specimens was used. The plating bath for copper electrodes deposition was composed of 1:1 mixture of freshly prepared solutions A and B. Solution A was composed of 12 g L−1 NaOH, 13 g L−1 CuSO4.5H2O, and 29 g L−1 KNaC4H4O6.4H2O. Solution B was 9.5 mL L−1 HCHO. Distilled water was used to prepare the plating solution. The copper coated specimens were removed from the electrolyte solution, cleaned with warm water, and rinsed with acetone.
In the group 2, PVD method, direct deposition was performed on the titanium alloy caps. For this, a copper target was used (99.999%). The caps are placed in the middle of the PVD chamber, then, the chamber was pumped down to a base pressure of 5x10-6 Torr. METAPLAS IONON MZR 323 PVD equipment (Metaplas Ionon GmbH, Gladbach, Germany) was used to deposit the coatings. Cathodic arc (Cu) was used to evaporate the metals and a current of 60 A was applied to get the CuNPs deposits.
In the Group 3, a gold deposit was first made on the caps, the deposition was made using argon (Ar) (99.999%) and Au Target (99.999%), the chamber was pumped down to a base pressure of 5x10-6 Torr before. A source of DC Sputtering Ion Magnetron Materials Science Inc was used. Later the technique thermal evaporation to deposit the Copper thin films on titanium alloys substrate was used to deposit copper on the caps with gold. A Vecco EB-PVD apparatus was used at a rate of 0.1 Å/sec for copper deposition.
Later the copper deposits were made using argon (Ar) (99.99%), a copper target at (99.999%), pressure of 5x10-6 Torr and 60 seconds as deposit time. A Vecco EB-PVD apparatus was used at a rate of 0.1 Å/sec for copper deposition. The System was modified for the evaporation of high purity copper wires (99.999%). The base pressure of the thermal evaporation system was better at 1.6 × 10−6 mbar. The samples no were heated before deposition. The characterization was performed through atomic force microscope (NT-MDT NTEGRA Prima) and was analyzed with WSXM software.
Particle size of the synthetized CuNPs was determined for all groups by analysis of the atomic force microscope (AFM). Results showed that group 1 had a particle size between 0.1-0.5 µm, these deposits presented a wide distribution of particle size and it was not possible to exactly determine them by AFM but by SEM Microscopy. Group 2 and 3 were characterized by a particle sizes in the range of 20-40 nm. The CuNPs coating obtained by distribution for the combined DC-Sputtering/PVD method had a narrower particle size distribution, compared to those deposits obtained only with PVD. This is because gold and copper have a face-centered crystalline structure (FCC), which facilitates the nucleation and growth process of CuNPs. AFM micrographs are shown in Figure 1.
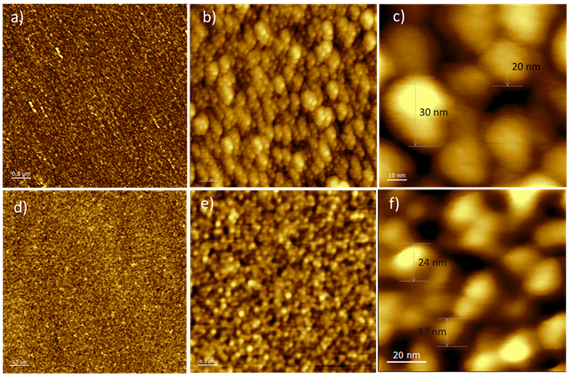
Figure 1 AFM Micrographs of CuNPs deposits on titanium alloys Caps using PVD (a-c) and DC-Sputtering/PVD (d-f). Micrographs obtained at different magnifications.
Antibacterial properties
Antibacterial properties of all groups were evaluated against an in vitro oral biofilm (Dentaid-1 plates) Process developed and described in detail by Sanchez MC. et al. (13) and Blanc V. et al. (14) In Summary, the bacterial inoculum was made by combining the following selected bacteria: S. oralis (103CFU /ml), S. gordonii (103CFU /ml), V. parvula (105CFU /ml), P. gingivalis (105CFU /ml) and P. intermedia (105CFU /ml) in a modified medium of brain-heart infusion (BHI) supplemented with mucin. A final volume of 3 mL of bacterial inoculum by test tube was obtained. The inoculum was grown by anaerobic incubation at 37ºC for 48 hours. After that, the caps (previously sterilized by autoclaved) were placed, one per test tube, and then were incubated at 37oC under anaerobic conditions for four days.
After the incubation time, three caps of each group were assessed by scanning confocal laser optical microscopy (CLSM) (Syto9 staining and observation at 10 x, 2.5 and 5 zoom) and the other 3 caps of each group were turned for 5 minutes in Eppendorf tubes with 1 mL of phosphate buffered saline (PBS) and seeded in plates containing a mixture of blood agar and Dentaid-1 plates in a 1:1 ratio (Figure 2). The condition of observation by CLSM were set as follow: immersion of the healing cap in staining in a Syto9 staining (Bacterial Viability Kit (Invitrogen, Carlsbad, CA) for 10 minutes at room temperature and in darkness; Laser output: 40%; 482nm laser line: 7% HyD3 detector, with 512 to 600 nm detection window; 100% Smart Gain. Pinhole 0.85 AU. Finally, a 10X objective with 2.5 and 5 zoom was used.
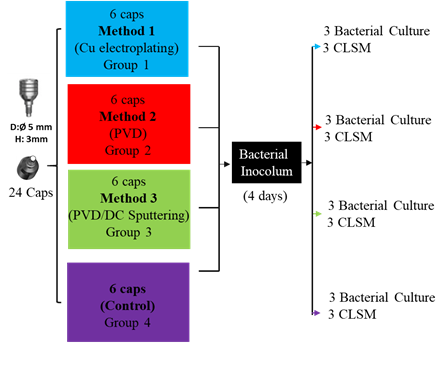
Figure 2 Diagram of the sample’s distribution prepared for bacterial culture, with their respective deposit methods.
On the other hand, to proceed with plate count method, 3 caps of each group were turned for 5 minutes in Eppendorf tubes with 1ml of PBS and seeded in plates with half blood agar and dentaid-1 plates, and were incubated at 37oC under anaerobic conditions for 2 days. All experiment were made in triplicates.
The microbial count showed that group 1 has the lowest bacterial load and bacterial aggregates, compared to the rest of the groups, 7,03E+06 versus. 9,77E+06 of group 2, 1,43E+07 of group 3, and 1,88E+07 of group 4 (Table 2). Total Microbial load by Group is shown in Figure 3. Also there was no count of P.gingivalis and P. intermedia in this group (Table 3).
Group 1 |
Group2 |
Group3 |
Group 4 |
7,03E+06 |
9,77E+06 |
1,43E+07 |
1,88E+07 |
Table 2 Total Microbial load by Group in Log 10 (CFU/mL)
|
Bacterial strain |
||||
|
S. oralis |
S. gordonii |
V. parvula |
P. gingivalis |
P. intermedia |
Healing Cap 1 |
2,80E+05 |
4,90E+05 |
3,03E+06 |
0,00E+00 |
0,00E+00 |
Healing Cap 2 |
1,23E+05 |
6,20E+04 |
4,20E+04 |
0,00E+00 |
0,00E+00 |
Healing Cap 3 |
9,00E+05 |
2,45E+05 |
1,86E+06 |
0,00E+00 |
0,00E+00 |
Group 1 average |
4,34E+05 |
2,66E+05 |
1,64E+06 |
0,00E+00 |
0,00E+00 |
Table 3 Average values of bacteria after 96 hours of incubation for the three healing caps of Group 1 in Log 10 (CFU/mL)
The microbial count showed that Group 2 (9,77E+06) has a similar bacterial load that group 3 (1,43E+07) however, differences in the disposition and size of bacterial aggregates were observed in the lower and inner part (i.e. area coinciding with the least amount of CuNPs) of the healing caps, in the outer part no aggregates were observed; what was observed was a lawn of bacteria attached to the surface (Table 4).
|
Bacterial strain |
||||
|
S. oralis |
S. gordonii |
V. parvula |
P. gingivalis |
P. intermedia |
Healing Cap 1 |
3,70E+05 |
1,85E+05 |
3,69E+06 |
1,80E+04 |
2,70E+05 |
Healing Cap 2 |
1,30E+05 |
1,00E+05 |
3,70E+05 |
6,50E+03 |
1,75E+05 |
Healing Cap 3 |
6,65E+05 |
7,10E+05 |
2,12E+06 |
1,15E+05 |
8,50E+05 |
Group 2 average |
3,88E+05 |
3,32E+05 |
2,06E+06 |
1,15E+05 |
4,32E+05 |
Table 4 Average values of bacteria after 96 hours of incubation for the three healing caps of Group 2 in Log 10 (CFU/mL)
The microbial count of group 3 showed similar quantity and distribution of bacterial aggregates to Group 2 (Table 5). Finally, the control group (Group 4), showed more bacterial load and aggregates than in the rest of the groups, as presented in Table 6.
|
Bacterial strain |
||||
|
S. oralis |
S. gordonii |
V. parvula |
P. gingivalis |
P. intermedia |
Healing Cap 1 |
7,80E+05 |
4,60E+05 |
3,87E+06 |
1,00E+02 |
6,30E+04 |
Healing Cap 2 |
4,35E+05 |
6,20E+05 |
4,20E+06 |
0,00E+00 |
0,00E+00 |
Healing Cap 3 |
3,30E+05 |
1,65E+05 |
2,21E+06 |
6,50E+04 |
1,09E+06 |
Group 3 average |
5,15E+05 |
4,153+05 |
3,43E+06 |
2,17E+04 |
3,84E+05 |
Table 5 Average values of bacteria after 96 hours of incubation for the three healing caps of Group 3 in Log 10 (CFU/mL)
|
Bacterial strain |
||||
|
S. oralis |
S. gordonii |
V. parvula |
P. gingivalis |
P. intermedia |
Healing Cap 1 |
2,10E+05 |
5,00E+04 |
2,10E+06 |
4,60E+04 |
9,20E+05 |
Healing Cap 2 |
5,10E+05 |
4,95E+05 |
2,64E+06 |
3,70E+04 |
9,35E+05 |
Healing Cap 3 |
5,85E+05 |
3,50E+05 |
8,10E+06 |
5,00E+04 |
1,80E+06 |
Group 4 average |
4,35E+05 |
2.98E+05 |
4,28E+06 |
4,43E+04 |
1,22E+06 |
Table 6 Average values of bacteria after 96 hours of incubation for the three healing caps of Group 4 in Log 10 (CFU/mL)
On the other hand, the observations made by CLSM in the other 3 caps (Figures 4a-4d) showed and confirmed the results of bacterial counts. A lower microbial load was observed in the caps of group 1 (Figure 4a), whereas, no differences can be observed between the caps of the Group 2 (Figure 4b), Group 3 (Figure 4c) and the control Group (Figure 4d). Interestingly, the lower microbial load found in the group 1 is due to the absence of periodontopathogenic and late colonizer bacteria such as P. gingivalis, and P. intermedia. Initial and early colonizer bacteria such as S. oralis, S. gordonii, and V. parvula are not affected (Table 7). S. oralis and S. gordonii are present in a similar amount in all 4 groups, and appear to be unaffected by the presence of CuNps (Figure 5).
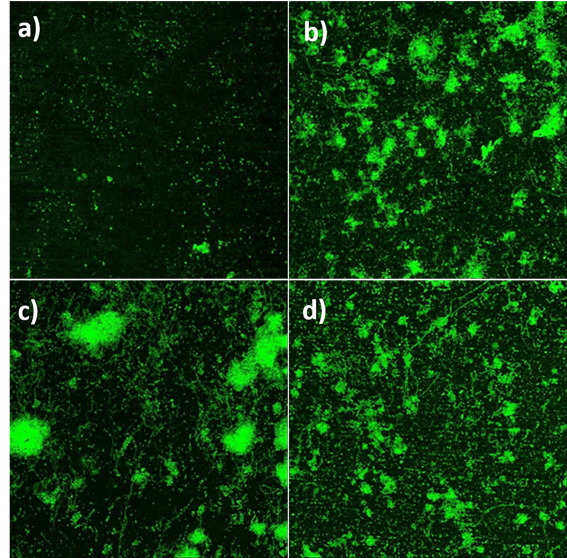
Figure 4 CLSM images of biofilm over healing caps. a) group 1, b) group 2, c) group 3, d) group 4. All images were taken at 5x magnification.
Strain |
Average microbial counts for the assessed groups (CFU) |
|||
Group 1 |
Group 2 |
Group 3 |
Group 4 (control) |
|
S. oralis |
4,34E+05 |
3,88E+05 |
5,15+05 |
4,35E+05 |
S. gordonii |
2,66E+05 |
3,32E+05 |
4,15E+05 |
2,98E+05 |
V. parvula |
1,64E+06 |
2,06E+06 |
3,43E+06 |
4,28E+06 |
P. gingivalis |
0,00E+00 |
4,65E+04 |
2,17E+04 |
4,43E+04 |
P. intermedia |
0,00E+00 |
4,32E+05 |
3,84E+05 |
1,22E+06 |
Table 7 Average microbial counts for the assessed groups in Log 10 (CFU/mL)
This pilot study describes the development of bacteria immersed in oral biofilm, in the presence of titanium alloy coated with CuNps.
The bacteria used in our study were the same used by Hwey et al.19 in a periimplantitis study, which showed that patients with periimplantitis have high levels of periodontal pathogens. However, in order to represent a large spectrum of microbial complex as described by Socransky et al.22 we chose for this study the use of S. oralis and S. gordonii (initial colonizer,23 yellow complex), V. parvula (early colonizer,23 purple complex), P.gingivalis (red complex, late colonizer23) and P. intermedia (orange complex, late colonizer23).
The antimicrobial activity of CuNps has been studied against different pathogenic microorganisms,1,2,4,9,16,17,21 for example, Sabatini et al.9 show a reduction of number of bacteria S. Mutans, in a polyacrylic acid coated copper iodide nanoparticles into dental adhesives. Ramyadevi et al.4 also showed antimicrobial activity against five bacterial strains and three fungal strains. Zhang et al. 2 And Liu et al.16 in others studies showed that no antibacterial activity was detected in their titanium (Ti) cooper (Cu) alloy and Ti-Cu alloy exhibited antibacterial property against E. coli and S. aureus. Further, their results concluded that Ti-Cu sintered alloys with different Cu contents exhibit very good cell biocompatibility.2
Recently, Amiri M et al.21 concluded that Nano-copper oxide used showed a high antimicrobial effect against the dental caries and also some effect on three species of candida. Thus, this Nanoparticles could be introduced as a candidate control agent for preventing dental caries or dental infections. It was thought that antibacterial mechanism is associated with the Cu ion released from the titanium copper alloy.
Our result showed a significant antibacterial activity of group 1, which could be attributed to the size and shape of CuNps, according to Sayes et al.24 Semisch et al.25 and Campoccia et al.26 The intrinsic factors of nanoparticles that show antibacterial activity are: Concentration, crystallinity, size (the smaller has greater bactericidal activity, especially for sizes <30nm), morphology (the triangular and sharper has greater activity than spherical), and high surface area, which may facilitate redox reactions either intra or extracellular leading to cell death. In the group 1, it was observed a size between 0.1-0.5 µm, and more heterogenic shape. In the group 2, it was observed size and shape more spherical and uniform, about 20-40 nm (Figure 1). The particle characteristics described above will also determine the intracellular bioavailability and thus the potential toxicity of the respective compounds. While copper ions enter the cell via transporters, nanoparticles may be internalized by endocytosis.24 So, one reason that would explain more bactericidal characteristic could be explain by the shape of CuNPs obtained by copper electroplating and the released of copper ion inside de plates. Another reason for more antibacterial activity would to the peeling of CuNPs observed in plates. Thus the copper ions of nanoparticles dissolved in the incubation medium may contribute to the bacterial cytotoxicity. It was observed a more effective antibacterial activity against late colonizer bacteria, like P.gingivalis, and P. intermedia (Table 3), which could be of interest since. On the other hand S. oralis and S. gordonii are present in a similar amount in all 4 groups, even more in the groups with CuNps than control, and appear to be unaffected by the presence of CuNps (Figure 5). This and that the negative control showed an unexpected number of bacteria, which, even when this study was done with duplicates, could be explained only by an experimental problem that require a larger sample size
We used two ways of add CuNPs because, the antibacterial activity would be significantly affected by the physical properties, such as, the poor adhesive strength in electrodeposition almost always leads to the failing of the antibacterial coating, which results in the peeling of antibacterial properties. For the PVD method, the antibacterial surface is normally very thin, with spherical shape and strongly adhered. Once the antibacterial surface is destroyed o peeling by some reasons, the antibacterial property would disappear.1 But this not, for us, desirable characteristic (peeling) could be a reason of this bactericidal effect observed on Group 1. Nonetheless is still desirable to develop a metal material, which has antibacterial activity in the whole alloy rather than on the surface, be stable with regards to their chemical and physical properties,5,9,12 and no peeling in addition, this peeling could generate some toxicity and a decrease of cell biocompatibility.24,27 Potential applications of metallic nanoparticles in dentistry require that nanoparticles interact well with oral tissues, in order to produce a sustained antibacterial effect on the time.17
In the same way that Campoccia et al, we believe that the tendency should be oriented to develop anti-infective biomaterials become a primary strategy to prevent medical device associated infections, and they can also be employed in the delivering of medical substances.28,29
Nevertheless, more studies are necessary for declare this observation, above all determine the reason why only CuNps obtained by certain methods were more bactericidal tan others and the reason why only some bacteria were affected.
Within the limitations of this study, it can be concluded that CuNps obtained by copper electroplating showed greater bactericidal effect that PVD methods, especially in periodontal pathogenic bacteria like P.gingivalis, and P. intermedia. More studies are necessary for corroborate this observation and better understand the reason why only CuNps obtained by certain methods were more bactericidal tan others and the reason why only some bacteria were affected. The antimicrobial properties exhibited by CuNps could be useful for develop anti-infective biomaterials become a strategy to control dental biofilm.
The authors would like to thank to the financial support of Alphabio tec ®, in the support of healing caps and Dentaid Laboratories in microbiological procedures, especially Dr. Rubén León. To Dr. Alberto Bezama From UFZ Leipzig. And to the Interdisciplinary Group of Advanced Nanocomposites (GINA) of the Department of Engineering Materials (DIMAT, according its Spanish acronym), Engineering School of the University of Concepción, for its laboratory of nanospectroscopy (LAB-NANOSPECT). MFM thanks CONICYT project: FONDEQUIP Project N°EQM150139, PIA/APOYO CTE AFB170007
The authors declare no conflict of interest.

©2021 Gómez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.