Journal of
eISSN: 2373-4345


Research Article Volume 6 Issue 1
1VTPCHEM PTY LTD, Australia
2Department of Orthodontics and Paediatric Dentistry, University of the Western Cape, South Africa
Correspondence: Tamara Perchyonok, VTPCHEM PTY LTD, Glenhuntly, Melbourne, 4215, Australia, Tel 61414596304
Received: December 28, 2016 | Published: January 10, 2017
Citation: Perchyonok T, Mulder R. Bio-active restorative materials as alternative pit and fissure sealants in pediatric and preventative dentistry: in vitroinvestigation. J Dent Health Oral Disord Ther. 2017;6(1):1-5. DOI: 10.15406/jdhodt.2017.06.00184
Background: It has been more than 40 years since the pit and fissure sealants were first used clinically. During this time, pit and fissure sealants have been shown to be effective in reducing the risk of occlusal caries.
Aim: The aim of the investigation is to further develop and evaluate a versatile designed chitosan based bio-active materials on for use as bonding free fissure sealant/fissure protectors on permanent dentition and evaluate remineralization/demineralization capacity of the materials through pH cycling, as well as shear bond strength etch and no etch prototype as well as measurement of Vickers hardness of the newly designed materials and compare the property with the commercially available standard.
Results: In general there was an increase in bond strength of the enamel treated with the modified Premise containing nanodiamond: chitosan materials compared to the bond strength of the conventionally bonded teeth. It is seen that release of phosphorus into the dematerializing solution (i.e. loss of phosphorus from the samples) showed larger amplitude (from 600.2mg to 101.3mg) than the uptake of phosphorus by the samples from the re-mineralizing solution (from 125.2mg to 66.1mg). Therefore, the treatment with chitosan seems to act more on the demineralization of tooth enamel with little effect on the remineralization process. Regarding the net phosphorus loss (net P loss), it can be seen that net demineralization occurs in all cases. However, the net amount of phosphorous released by the control group samples was significantly higher than those groups treated with chitosan. The net P loss for the control group was 475 mg of P, whereas the groups containing chitosan had a net P loss in the range of 30-182mg. When a 1mm layer is assessed all the materials including the Premise control indicated a conversion of above 96%, which is the upper limit of the conventional fissure sealant material which makes the newly designed bioactive materials suitable for the application as fissure sealant materials. The important aspect of any newly designed/ developed restorative material is cytotoxicity as Grobler et al.1 investigated the cytotoxic effect of nanodiamonds and also the effect of the incorporation in a dental material (Premise), who found a higher shear bond strength (p < 5%) after 3 months of Premise treated with nanodiamonds, chitosan, cyclodextrin (CD) and combinations thereof than for the control Premise. The sequence for the Vickers hardness was: CD (32.5) < nano (34.8) < CD Nano (38.8) < Premise (39) < Chitosan Nano (42.2). Nanodiamonds (92%) and the combination of chitosan + nanodiamonds (93%) showed little cytotoxicity. The shrinkage was lower for all the additions than for Premise alone.
Conclusion: All modified Flowable bio-active materials can be further developed in effective fissure sealant material based on the acceptable in vitro results and cytotoxicity data.
Fissure sealants are recommended to be applied soon after the tooth eruption, mainly at the level of the first permanent molars. The additional benefits of the application of bioactive fissure sealant materials lies in the build-in functionality of these materials to chemical attack in oral cavity as well as additional antibacterial action.
It has been more than 40 years since the pit and fissure sealants were first used clinically. During this time, pit and fissure sealants have been shown to be effective in reducing the risk of occlusal caries,2 and their success largely depends on the long term retention and tight micromechanical adhesion to enamel surfaces.3 Resin based sealants can be classified as either filled or unfilled to the filler contents. There is a great deal of controversy regarding the most appropriate type for pit and fissure sealant. Droz et al.4 reported that filled sealant is less likely to completely fill a fissure than an unfilled sealant because a less viscous sealant would penetrate the fissure more deeply.5 Barnes et al.6 however, reported that the viscosity and flow properties of fissure sealants did not affect their sealing ability several studies have also shown similar penetration capability and retention in the 2 sealant types.6 Recently, flowable composites have been marketed as pit and fissure sealants with the view that Flowable composites have a higher wear resistance. One study reported that 20% of practitioners used flowable composites as sealants and 29% of the practitioners used bonding systems before applying flowable composites or compomers.7 The same study also reported that no practitioners used bonding agents with classical sealants. If flowable composites have a comparable bonding quality with enamel without using a bonding system, they can be recommended for widened occlusal fissures with the benefit of a better abrasion resistance than conventional filled sealants. Chitosan and modified chitosans are interesting candidates in this respect. Chitosan, a natural linear bio-polyamino saccharide is obtained by alkaline deacetylation of chitin.8 This material is also biocompatible and biodegradable. It is positively charged and combines with the bacterial cell wall and membrane with bacteriostatic and bactericidal results. Muzzarelli et al.9 Phosphorus chemical analysis was carried out by a spectroscopic method described elsewhere.10
A random experimental design with 5 groups (Premise, 10%CNO, 10%CNB, 10%CNU and 10%CNC) containing 3 pieces each was used, and all groups were submitted to the pH cycling. One group (blank) was prepared without exposed dental enamel. This was achieved by completely coating the piece with acid resistant varnish. The blank was added to the experiment in order to verify that the materials employed in the pieces did not promote release or absorption of calcium and phosphorus, therefore, having no effect in quantitative analyses of these ions. Two other groups (control) did not contain chitosan in the samples, and were cycled in solutions DE4.0 and DE4.8, respectively. Bioactive modified nano-diamond chitosan containing Flowable composites were applied on enamel by means of a small brush and cured for 20 seconds. After a certain period (time of chitosan action), the sample was submitted to pH cycling. The results were analyzed using the software Prism. Averages were compared by the Student test in the 95% confidence level. The demineralization and the remineralization processes were followed separately by phosphorous chemical analysis. The amount of phosphorous released (DE) or absorbed by the dental specimen was calculated cycle by cycle. The sum of 5 cycles is represented in the Figure 1, separately for the demineralization and the remineralization process for the different group of specimens studied. The Figure 1 shows also the net phosphorous loss (net P loss=DE-RE).
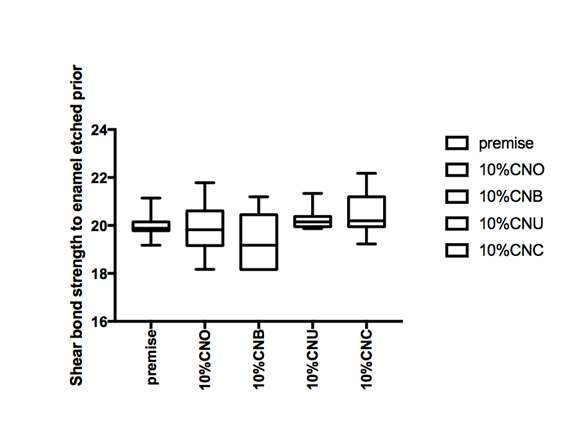
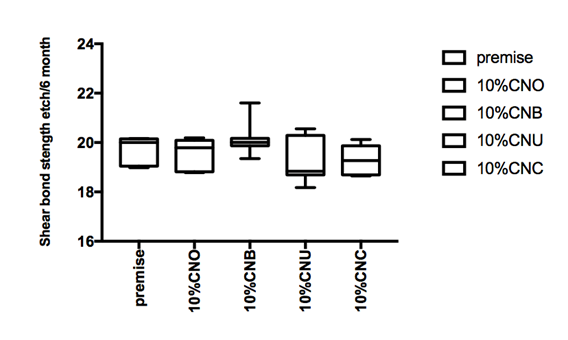

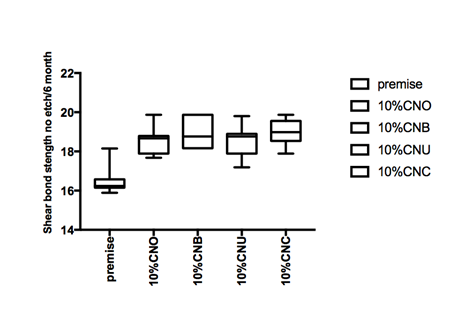
Figure 1 Hear bond strength in mPa A: Enamel bonding with etching after 24 hours debonding; B: Enamel bonding with etching after 6 month debonding. C: shear bond strength to enamel bonding with no etching after 24 hours debonding; D: shear bond strength to enamel bonding with no etching after 6 month deboning.
Shear bond strength etch and no etch prototype
Extracted human molars were used within 2 months of storage in water containing thymol crystals. Only undamaged teeth were selected. The roots of the teeth were removed and all the occlusal enamel exposed. The teeth were embedded in 10mm length PVC (Consjit Tubing, SA PVC, JHB, RSA) pipes with cold cure acrylic resin so that the exposed enamel is projected well above the acrylic and the dentin then thoroughly washed under tap water. Two modified flowable materials composite studs each with an internal diameter of 2.6mm and height of 1mm were bonded to the enamel surface of each tooth via etching with orthophosphoric acid (37%) prior to applying and curing with modified flowable material or no etching used prior to applying the modified flowable material. In this way, 30 tooth samples (each containing two studs) were prepared and divided at random into 5 groups of 8 each. The teeth were stored in a solution of artificial saliva. After 24h, the shear bond strength of one stud of each tooth was tested for failure (Zwick Universal Testing Machine, Germany) by means of a knife-edged rod at a crosshead speed of 0.5mm/min. The other stud was tested after 6 months. All data were analyzed using the non-parametric ANOVA test.
Vickers hardness measurement
Spectrum 800mW/cm2 halogen curing light (Dentsply) was used to cure the test samples. The output was continually monitored with a cure right (Caulk) radiometer. The surface microhardness was determined with a Vickers Hardness tester (Zwick-Roell durometer, ZHV1/2 Micro-Vickers, Italy) using Vickers diamond indenters HV0.5 (500gf) load with a dwell time of 15 seconds.11 The specimens tested were the Premise control (Kerr, California, USA) as well as the Premise modified by 10% chitosan: nanodiamonds: oblepicha, 10% Chitosan: nanodiamonds: brasilian propolis, 10% Chitosan: nanodiamonds: uruguay propolis, 10%Chitosan: nananodiamonds: coparilba. Five samples were prepared for each material combination under laboratory conditions for the Vickers Hardness. A standard Teflon mould placed between two myalar strips and two glass slabs were used with a diameter of 5.0mm and a height of 2.0mm. The light cured samples were placed in the specimen holders on a moist paper towel and kept at T 34˚C±1 for 48 hours in a temperature controlled incubator. The five indentations of the five samples were taken and an average calculated for each material. Five indentations were made in accordance with ASTM E384: Standard Test Method for Knoop and Vickers Hardness of Materials. The distance between indentations were 2.5 the indentation size. The distance from the centre of the indentation to the edge of the specimen was also 2.5 the indentation size. The average of the five indentations was taken as the reading for each sample. The same procedure was followed to assess the Vickers hardness of the bottom of the specimen.
Figure 1a and Figure 1b gives the shear bond strength values (MPa) after 24 hours and after 6 month of storage of samples in artificial saliva, respectively, using conventional fissure sealant protocol discussed in the experimental section. In general there was an increase in bond strength of the enamel treated with the modified Premise containing nanodiamond: chitosan materials compared to the bond strength of the conventionally bonded teeth. An increase in the shear bond strength was also previously reported12 for chitosan containing hydrogels. Interestingly the increase in bond strength was also observed in the groups of hydrogen peroxide exposed samples suggesting that there additional benefits associated with nanodiamond: chitosan: bioactive system is in need of further investigations.1 The results of this study suggests that the optimum results for the strengthening of enamel can be achieved throughout the immediate treatment with bioactive: chitosan: nanodiamond with the increase of dentin bond strength. Also, impressively an almost immediately after the corresponding modified flowable material treatment and proceeding with bonding procedures is recommended with the significant increase in bond strength. The additional advantage of the system may suggest that, chitosan: bioactive and nano-diamond interaction with crystalline hydroxyapatite structure of the enamel layer increases the dentin bond strength observed especially in the case of the direct bonding between the hydrogel and the enamel interface. The additional benefit of using chitosan: antioxidant system as a bonding/pre-bonding to enamel and dentin system lies in its ability to show favorable immediate results in terms of bonding effectiveness as well as the durability of resin-enamel bonds for a prolonged time (up to 6 months).12
Remineralization/de-mineralization results and total P loss: effects of chitosan on de-remineralization of dental enamel
The influence of chitosan can also clearly be seen in Figure 2. This figure shows the release and uptake of phosphorous after 5 cycles of de-remineralization, according to the chemical analysis of the solutions. It is seen that release of phosphorus into the demineralizing solution (i.e. loss of phosphorus from the samples) showed larger amplitude (from 600.2mg to 101.3mg) than the uptake of phosphorus by the samples from the remineralizing solution (from 125.2mg to 66.1mg). Therefore, the treatment with chitosan seems to act more on the demineralization of tooth enamel with little effect on the remineralization process. Regarding the net phosphorus loss (net P loss), it can be seen that net demineralization occurs in all cases. However, the net amount of phosphorous released by the control group samples was significantly higher than those groups treated with chitosan. The net P loss for the control group was 475 mg of P, whereas the groups containing chitosan had a net P loss in the range of 30-182mg.
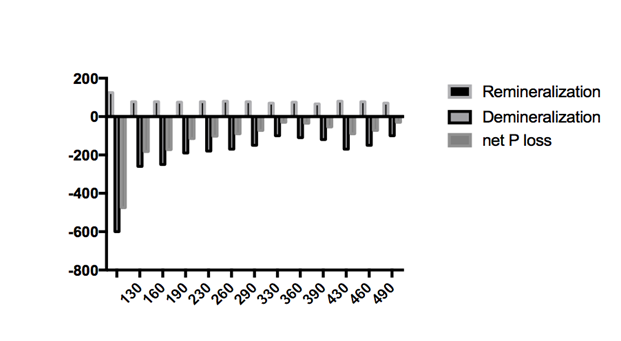
Figure 2 Cumulative phosphorous content of demineralization (expressed as negative number) and remineralization solution and their difference (net P loss) after 5 cycles for each group of samples investigate.
Microhardness of the materials as potential fissure sealants
A One Way analysis of variance (ANOVA) was carried out to investigate if statistically significant differences existed between the various materials. The Vickers hardness values for the top vs bottom is tabulated for a material layer of 1 mm. The ratio between the top and the bottom values for the 2mm layer clearly indicate that there are insufficient cure of the modified materials 10% Chitosan: nanodiamonds: Uruguay propolis as well as the 10% Chitosan: Nano-diamonds: Copaiba oil which makes the materials not suitable for the conventional restorative materials . Although a sufficient cure above 80% were reached for the remaining Premise modifications, the Vickers hardness was reduced in relation to the control Premise material. When a 1mm layer is assessed all the materials including the Premise control indicated a conversion of above 96% , which is the upper limit of the conventional fissure sealant material which makes the newly designed bioactive materials suitable for the application as fissure sealant materials. Evaluation of micro-hardness is widely used as a test to assess the curing of the composites and the efficiency of curing light sources.13–15 The criteria of the bottom to the top hardness ratio of 0.80-0.90 have been used as a predictor for adequate depth of curing at a specific sample thickness. This criteria means that the ratio of the bottom to top surface micro-hardness is 80% or more will indicate adequate curing.16,17 Zorzin et al.18 proposed that the optimum curing of a material can only be considered should there exist no decrease below 80% of micro-hardness with increasing material depth. The filler size and content of dental composites affect the depth of light penetration and it has a direct relationship to the depth of cure.19 The additional modifications do change the filler loading as well as the viscosity and should be considered when indications for these modified materials are sought. All the material combinations tested can however be used in 1mm layers that will include liners, fissure sealants and sealant restorations that extend into enamel. The important aspect of any newly designed/ developed restorative material is cytotoxicity as Grobler et al.20 investigated the cytotoxic effect of nanodiamonds and also the effect of the incorporation in a dental material (Premise), who found a higher shear bond strength (p < 5%) after 3 months of Premise treated with nanodiamonds, chitosan, cyclodextrin (CD) and combinations thereof than for the control Premise. The sequence for the Vickers hardness was: CD (32.5) < nano (34.8) < CD Nano (38.8) < Premise (39) < Chitosan Nano (42.2). Nanodiamonds (92%) and the combination of chitosan + nanodiamonds (93%) showed little cytotoxicity. The shrinkage was lower for all the additions than for Premise alone.21–23
In conclusion, all modified flowable bio-active materials can be further developed in effective fissure sealant material based on the acceptable in vitro results and cytotoxicity data.
None.
None.
The authors declare there is no conflict of interests.

©2017 Perchyonok, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.