Journal of
eISSN: 2373-4396


Case Report Volume 12 Issue 4
Cardiology Devision, Assuta Ashdod University Medical Center, Israel
Correspondence: Ella Yahud, Cardiology Division, Assuta Ashdod University Hospital, 7 HaRefua Street, Ashdod 7747629, Israel, Tel +972523935075, Fax +972737436226
Received: June 17, 2019 | Published: August 30, 2019
Citation: Yahud E, Rahkovich M, Laish-Farkash A. Resolution of anosmia and urinary incontinence after ablation of supra-ventricular tachycardia. J Cardiol Curr Res. 2019;12(4):100-102. DOI: 10.15406/jccr.2019.12.00449
Rationale
Supraventricular tachyarrhythmias (SVT) may cause a significant impairment of quality of life. The smell alterations may appear in association with SVT due to side effects of antiarrhythmic drugs1 but may as well be a manifestation of another underlying disease.2–4
Patient concerns
In this case report, we describe a case of a 51-year-old female which developed loss of the sense of smell (anosmia) and urine incontinence around the time when she was diagnosed with supraventricular tachycardia. After radiofrequency ablation of atrioventricular nodal re-entry tachycardia (AVNRT) and atrial tachycardia, her ability to smell had recovered and urinary incontinence had improved dramatically. During 18-month follow-up, she reported no complaints of smell anymore. Possible underlying mechanisms are discussed.
Lessons
Our case report describes a patient with a supraventricular tachyarrhythmia who suffered reversible anosmia and urine incontinence which resolved after radiofrequency ablation. Anosmia in our case report had led to a large spectrum of underlying pathologies to consider. The most likely causes of smell disorder in this case were typical aura without headache –a rare type of migraine or somatization disorder. Catheter ablation may have resulted in the reversal of autonomic imbalance causing improvement and later elimination of anosmia.
Keywords: supraventricular arrhythmia, smell, radiofrequency ablation, migraine, Parkinson’s disease, anosmia, atenolol, urine incontinence
SVT, supra-ventricular tachycardia; AV, atrio-ventricular; AT, atrial tachycardia; CL, cycle length; PD, parkinson's disease; AF, atrial fibrillation
A 51years old female with a previous history of hypertension, hyperlipidemia and celiac disease presented to our clinic with complaints of palpitations for the last 5years. Her chronic medications were atenolol 12.5mg once daily and pravastatin 40mg once daily.
The palpitations were sudden in onset and were terminated by vagal maneuvers. The patient reported no shirt flapping or neck pounding. Her physical examination and echocardiographic exam were normal as well as her blood tests, including thyroid function.
The ECG documented narrow complex short RP tachycardia with ventricular response of 140bpm. The patient noticed that around the time that the symptoms of palpitations appeared for the first time, she had lost the sense of smell and started to suffer from urinary incontinence but didn’t search for medical advice. She was assessed in our clinic and decision was made to proceed with electrophysiology study. The study showed dual atrio-ventricular (AV) node physiology. Supra-ventricular tachycardia (SVT) (Figure 1) was induced at a cycle length (CL) of 380ms with ventriculo-atrial interval of 35ms, and entrainment maneuvers supported typical AV node re-entry tachycardia as the working diagnosis. Slow pathway ablation was performed with successful outcome. During isuprel administration and atrial pacing incessant atrial tachycardia (AT) with two different CL of 360 and 320ms was provoked (Figure 2).
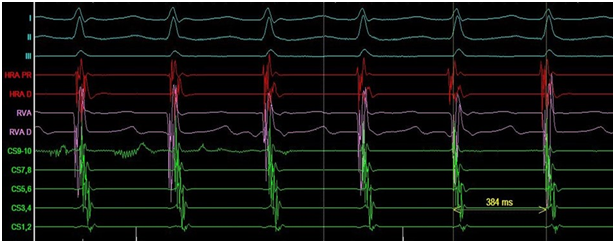
Figure 1 Typical atrioventricular nodal re-entry tachycardia (AVNRT).
Electrophysiological study: I, II, III- ECG leads; HRA- high right atrium; RVA- right ventricular apex; D- distal electrode, PR- proximal electrode; CS- coronary sinus.
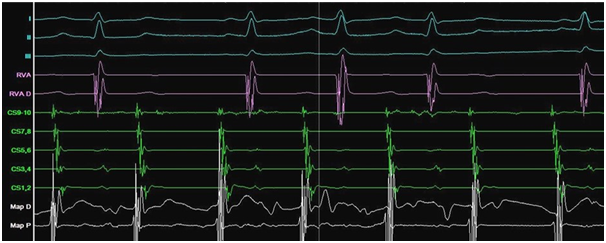
Figure 2 Incessant atrial tachycardia.
Electrophysiological study: I, II, III- ECG leads; RVA- Right ventricular apex; CS- coronary sinus; P- proximal electrodes; D- distal electrodes; Map- mapping catheter.
Carto mapping detected 2 foci of AT origin: crista terminalis and right atrial anterior septum (Figure 3). These two foci were ablated and were non-inducible at the end of the procedure. The patient was further discharged with a recommendation to continue treatment with atenolol 12.5mg once daily. She was then followed-up for 18 months after the ablation in our outpatient clinic. No SVT was detected in repeated continuous Holter monitoring during the follow-up period. The patient reported no palpitations; moreover, resolution of anosmia and significant improvement of urinary incontinence occurred immediately after the ablation and ever since.
We present a unique case of SVT-induced anosmia and resolution of anosmia after radiofrequency ablation of SVT. To the best of our knowledge, this is the first report of such an association. There are several potential causes of anosmia described in the literature. Generally, anosmia is classified as either congenital or acquired.
Congenital anosmia can occur sporadically or be associated with hereditary genetic disorders such as congenital insensitivity to pain, Kallmann syndrome (hypogonadotropic hypogonadism, anosmia, osteoporosis), CHARGE syndrome (coloboma of the eye, central nervous system anomalies, heart defects, atresia of the choanae, retardation of growth and/or development, genital and/or urinary defects, ear anomalies and/or deafness and abnormally shaped ears).3 Acquired anosmia may have multiple causes: inflammation in the nasal pathways,4 head trauma, olfactory groove tumors, neuro-degenerative disorders (Parkinson's disease, Alzheimer's disease, Lewy body disease), aging, psychiatric disorders, migraine equivalent, somatisation or depression. Anosmia has been linked to several drugs side effects. The most relevant drugs which are used in treating arrhythmias are metoprolol,1 digoxin5 and amiodarone.6
Our patient did not take any of the above mentioned drugs. Moreover, anosmia was resolved after the ablation while atenolol usage was continued; Impaired sense of smell as well as atrial fibrillation7,8 may occur in the earliest stages of Parkinson's disease (PD) and may precede motor symptoms by several years. The potential mechanism is an impairment of nerve cell growth in the region of olfactory bulb due to dopamine depletion which occurs in PD.2 The relative young age, the association with SVT timing, and the absence of motor symptoms years after appearance of anosmia make the diagnosis of PD unlikely in our patient; another possible cause of anosmia in this patient is a migraine aura. Aura can appear at some point in 38% of patients with migraine headaches. Moreover, typical aura without headache occurs in 4% of patients with diagnosis of migraine. Aura may present as olfaction‐related symptoms such as olfactory hallucinations (6.2%), phantosmia (4.4%), cacosmia/euosmia (2.6%).9 There is evidence to support a beneficial effect of atrial fibrillation (AF) ablation on duration and intensity of pain in migraine.10
Autonomic dysfunction seems to be involved in the pathophysiology of both migraine and SVT. Concomitant sympathetic hyperfunction and parasympathetic hypofunction was reported to be present in patients with migraine.11 Catheter ablation of AF decreases an efferent sympathetic nerve activity and stimulates postganglionic efferent parasympathetic fibers.12 Therefore, catheter ablation might play role in the reversal of autonomic imbalance causing elimination or improvement of clinical symptoms of migraine in AF patients.13 Its effect during SVT ablation may be similar but was not proved yet;
It is known that olfactory symptoms can be one of obscure manifestations of somatization and depressive disorders.14 It seems reasonable that improvement in quality of life and mood after SVT ablation may underlie the improvement of the olfactory symptoms in our patient.
As described previously,15 paroxysmal SVT might cause polyuria, increasing the urinary flow by twice at the end of SVT episode. The underlying mechanisms include increased release of atrial natriuretic peptide related to left atrial strain, increased anti diuretic hormone plasma level and increased levels of prostaglandin E2 stimulated by anti-diuretic hormone, similar to the mechanisms that underlie polyuria in AF patients.16 Successful ablation of SVT may therefore lead to significant improvement in polyuria and urinary incontinence, as described in our patient.
We report a unique case of resolution of anosmia and urinary incontinence associated with successful SVT ablation. Further studies investigating the underlying mechanisms are needed.
None.
The authors declare that there are no conflicts of interest.

©2019 Yahud, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.