Journal of
eISSN: 2373-4396


Research Article Volume 12 Issue 5
Cardiology Department, Faculty of Medicine, Zagazig University, Egypt
Correspondence: Dr. Mohammad Gouda, MD. Assistant Professor of Cardiology, Postal Address: Egypt, Zagazig, Zagazig University, Faculty of Medicine, Cardiology Department, 44519, Egypt, Tel +21019693393, Fax +20552357770
Received: August 16, 2019 | Published: September 23, 2019
Citation: Gouda M, Hassan ASAM, Abdel-Alwahab MF. Prediction of left ventricular systolic dysfunction after 6 months of aortic valve replacement in patients with chronic severe aortic regurge. J Cardiol Curr Res. 2019;12(5):112-116. DOI: 10.15406/jccr.2019.12.00451
Introduction: LVEF as a surrogate for myocardial performance is problematic in chronic AR as it is augmented by increased preload. Therefore, we need noninvasive parameters to assess myocardial function in altered loading conditions. We aim to assess the validity of new echo-Doppler indices for prediction of postoperative LV dysfunction 6 months after AVR.
Methods: We recruited 20 patients with severe isolated AR with LVEF >50%, prepared for AVR. Echocardiographic examination was done 48hours before and 6 months after AVR. Early diastolic driving force "DF", Global longitudinal strain "GLS" and Left ventricular ejection fraction "LVEF" by modified biplane Simpson method were measured. Our patients were classified according to postoperative LVEF into Group A (15 cases); normal (postoperative LVEF≥50%) and Group B (5 cases); postoperative LV systolic dysfunction (postoperative LVEF<50%).
Results: preoperative DF was 0.20±0.12 Newton in group A while it was 0.66±0.28 Newton in group B, this difference was statistically significant (t=3.5, p<0.05). EF was 62.73±6.64% in-group A while it was 53.2±2.77% in-group B, this difference was statistically highly significant (t=4.50, p<0.001). GLS was -18.95±2.55% in-group A while it was -12.56±2.04% in group B, this difference was statistically highly significant (t=5.04, p<0.001). On plotting the ROC curves, it was clear that preoperative GLS and DF are strong predictors of post-operative systolic dysfunction in such cases.
Conclusion: Preoperative GLS and DF seem to be independent predictors for postoperative LV systolic dysfunction after AVR for chronic severe AR.
Keywords: aortic regurgitation, aortic valve replacement, color doppler, diastolic function
AVR, severe aortic valve regurgitation; LV, left ventricular; LVFR, Left ventricular ejection fraction; GLS, global longitudinal strain; DT, deceleration time; ROC, receiver operating characteristic; DF, driving force; LRA, logistic regression analysis
The natural history of chronic severe aortic valve regurgitation (AR) is characterized by a prolonged period of preserved global left ventricular (LV) systolic function and compensated eccentric hypertrophy, which insidiously gives way to myocardial decompensation.1 In its early stages, myocardial dysfunction may be reversible once implantation of a mechanical or bioprosthetic aortic valve eliminates the volume load. If left untreated, myocardial dysfunction becomes irreversible, a condition associated with high rates of morbidity and mortality.2 The choice of optimal timing of aortic valve replacement (AVR) in patients with AR often poses a dilemma for clinicians. If AVR is performed at a time when LV function is normal, then the patient may be exposed unnecessarily to the potential hazards that accompany prosthetic valves. However, if surgical correction of the volume overload state is delayed excessively irreversible cardiac dysfunction may have already occurred and the outcome may be unsatisfactory.3 The decision is to when to intervene for chronic AR depends primarily on the symptomatic status of the patient and the potential for normalization of ventricular and myocardial performance after AVR.4 Measuring LVEF as a surrogate for myocardial performance is problematic in the setting of chronic AR because LVEF is augmented by increased preload and is therefore limited in detecting subtle myocardial dysfunction. Thus, there is a clinical need for quantitative noninvasive parameters to assess myocardial function in the setting of altered loading conditions and to predict postoperative LV systolic dysfunction.5 The aim of the study is to assess the validity of new echo-Doppler indices for prediction of postoperative LV dysfunction 6 months after AVR.
Methods
This study had been carried out in Cardiology Department, Zagazig University. It included 20 patients with severe isolated aortic regurgitation with LVEF >50%, prepared for AVR. We excluded patients with other significant valve disease (more than mild degree), associated congenital heart disease, and coronary artery disease with a significant stenosis, previous cardiac surgery and atrial fibrillation. Transthoracic echocardiographic examination was done before and 6 months after AVR. The following measures were selected for analysis:
Vena contarcta width, JET width, AR pressure half time. (Severe AR was diagnosed when vena contracta > 0.6 cm, JET width > 65 % of LVOT, PHT <200milliseconds).6
Performed 48h before & 6 months after AVR.
The early diastolic driving force calculated from the equation: DF=0.004E2/DT Newton.7 E wave (early diastolic mitral inflow velocity), DT (deceleration time) measured by pulsed wave Doppler imaging at mitral leaflet tips in 4-chamber view as the time-interval between peak of E wave and the point where the descending limb touches baseline.
Global longitudinal strain by speckle tracking; GLS evaluates the shortening and lengthening of the myocardial wall, measured from the three apical views (long axis, two and four chamber views): GLS was calculated by averaging the peak strain values of 17 segments.8 Normal regional peak systolic strain in the longitudinal direction is approximately −18 %.9
Ratio of early diastolic mitral inflow velocity (measured by pulsed wave Doppler imaging with sample volume set at the mitral leaflet tips in four chamber view) to early diastolic mitral annular velocity (measured by pulsed wave tissue Doppler imaging with sample volume set at the septal, lateral mitral annulus in four chamber view).
Left ventricular ejection fraction by modified biplane method of Simpson. 2 orthogonal views—apical four-chamber and apical two-chamber—and manual tracing of endocardial borders manually traced at end systole and end-diastole are needed.10 It is calculated from the formula: LVEF=(LVEDV-LVESV) x 100/LVEDV. Normally it is 50-70%, LV systolic dysfunction is said if LVEF<50 %.11
The patients were classified according to postoperative LV systolic function (postoperative LVEF) into:
Statistical analysis
Statistical presentation and analysis of the present study was conducted, using the mean, standard deviation, paired (T) test, independent t test, and chi square by SPSS V 20. Regression analysis was performed to determine the independent predictors of postoperative LV systolic dysfunction. Receiver operating characteristic (ROC) curve analysis was performed to determine cutoff values that predict postoperative LV systolic dysfunction. P value<0.05 was considered as statistically significant.
Demographically; we had 11 female patients (55%) and 9 male patients (45%). In group A, we had 8 females and 7 males while in group B we had 3 females and 2 males, there was no significant difference concerning the gender between both groups (X=0.09, p=0.77). The overall age of our study cases ranged from 19-45years with mean 32.55±7.8years. The age of cases in-group A was 29.80±6.74 years while in-group B it was 40.8±4.02 years, that represents a statistically significant difference (t=-3.41, p=0.003) Table 1.
|
Characteristics |
Age |
(t) |
P |
|||
|
Mean±SD |
||||||
|
Group A (n=15) |
29.80±6.74 |
-3.41 |
0.003* |
|||
|
Group B (n=5) |
40.8±4.02 |
|||||
|
Characteristics |
Female |
Male |
χ2 |
p |
||
|
No |
% |
No |
% |
|||
|
Group A (n=15) |
8 |
53.3 |
7 |
46.7 |
0.09 |
0.77 NS |
|
Group B (n=5) |
3 |
60 |
2 |
40 |
||
Table 1 Differentiation of patients according to demographic characteristics in relation to study groups
Etiologically; 55 % of our cases were rheumatic, 25% had Bicuspid AV and finally 20% of them had aneurysm of ascending aorta. There was no statistical significant differences between cases in both groups (X=0.09, p>0.05).
Concerning the preoperative measured Echo-Doppler indices, the driving force (DF) was 0.20±0.12 Newton in group A while it was 0.66±0.28 Newton in group B this difference was statistically significant (t=3.5, p<0.05). Regarding E/e', it was 11.95±5.52 in-group A while it was 21.12±3.43 in-group B, this difference was statistically highly significant (t=4.38, p<0.001). Concerning the EF, it was 62.73±6.64% in-group A while it was 53.2±2.77% in-group B, this difference was statistically highly significant (t=4.50, p<0.001). Lastly, GLS, it was -18.95±2.55% in group A while it was -12.56±2.04% in group B, this difference was statistically highly significant (t=5.04, p<0.001) Table 2.
|
Variable |
Group A (n= 15) |
Group B (n= 5) |
Test |
P |
|
Driving force |
0.20 ± 0.12 |
0.66 ± 0.28 |
3.5 |
<0.05* |
|
E/è |
11.95 ± 5.52 |
21.12 ± 3.43 |
4.38 |
<0.001** |
|
Ejection fraction |
62.73 ± 6.64 |
53.2 ± 2.77 |
4.5 |
<0.001** |
|
Global longitudinal strain |
-18.95 ± 2.55 |
-12.56 ± 2.04 |
5.04 |
<0.001** |
Table 2 Comparative study of preoperative echocardiographic indices between both groups
Correlation studies: There was a highly significant negative correlation between post-operative Ejection fraction and pre-operative GLS (R=-0.901, P<0.001), preoperative DF (r=-0.852, p<0.001) and pre-operative E/è (r=-0.806, p<0.001). In addition, there was significant negative correlation between post-operative Ejection fraction and ageing (r=-0.601, p<0.05) while there was a significant positive correlation between post-operative Ejection fraction pre-operative Ejection fraction (r=0.641, p<0.05) Table 3, Figure 1.
|
Pre-operative indices |
Postoperative LVEF |
|
|
r |
p |
|
|
DF |
-0.852 |
<0.001** |
|
E/è |
-0.806 |
<0.001** |
|
EF |
0.641 |
<0.05* |
|
GLS |
-0.901 |
<0.001** |
|
Age |
-0.601 |
<0.05* |
Table 3 Correlation of post-operative LVEF with other parameters
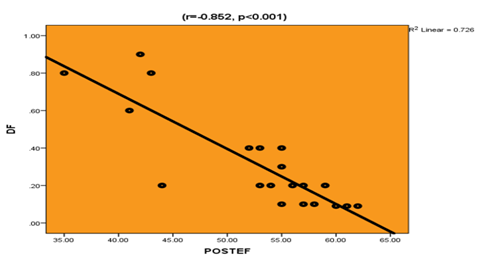
Figure 1 Correlation of post-operative left ventricular ejection fraction and pre-operative driving force.
On plotting the ROC curves, it was clear that preoperative GLS (AUC=99%, p<0.001, cut off value=-14%), preoperative DF (AUC=89%, p<0.05, cut off value=0.5 newton) and preoperative EF (AUC=87%, p<0.05, cut off value=55%) are strong predictors that can predict the occurrence of post-operative systolic dysfunction in such cases Table 4, Figures 2 & 3.
|
|
Cut off |
AUC |
p |
95% CI |
Sensitivity |
Specificity |
Accuracy |
|
DF |
0.5 |
0.89 |
<0.05* |
0.69 – 1.09 |
80 |
100 |
95 |
|
E/è |
18 |
0.67 |
>0.05 |
0.36 – 0.96 |
80 |
59.7 |
60 |
|
EF |
55 |
0.87 |
<0.05* |
0.72 – 1.03 |
80 |
73.3 |
75 |
|
GLS |
-14 |
0.99 |
<0.001** |
0.95-1.02 |
80 |
100 |
95 |
Table 4 Reliability data of different preoperative Echo Doppler indices as predictors for postoperative Systolic dysfunction
DF, Driving force; EF, Ejection fraction; GLS, Global longitudinal strain
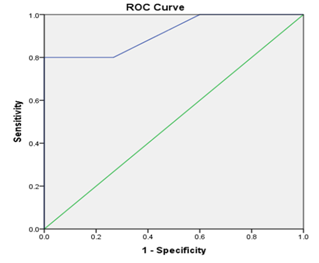
Figure 3 ROC curve of preoperative Driving force (DF) as a predictor of post-operative systolic dysfunction.
In order to detect the strongest predictor of post-operative systolic dysfunction in our cases, we performed the logistic regression analysis (LRA). It was clear that preoperative GLS was the strongest predictor of post-operative systolic dysfunction (R=-0.09, p<0.001) followed by preoperative DF (R=0.98, p<0.05) Table 5.
|
Variables |
Regression Coefficient |
SE |
p |
|
DF |
0.98 |
0.37 |
<0.05* |
|
E/è |
0.01 |
0.02 |
>0.05 |
|
EF |
-0.03 |
0.01 |
<0.05* |
|
GLS |
-0.09 |
0.02 |
<0.001* |
Table 5 Logistic regression Analysis of significant variables (different preoperative Echo Doppler indices) for postoperative systolic dysfunction
Current guidelines indicate surgery in symptomatic patients with severe AR or, in the case of asymptomatic patients, surgery is indicated when LV function is impaired (EF<50%) and should be considered if LV end-diastolic diameter (LVEDD) is >70mm or LVESD is >50mm (or >25mm/m2 BSA in patients with small body size). Left ventricular systolic dysfunction is initially reversible with surgery. However, AVR should be performed before onset of irreversible LV dysfunction. Despite these indications, the optimal timing of cardiac surgery for chronic AR remains challenging, because of systolic dysfunction may precedes symptom onset in more than one-fourth of patients with this condition. In addition, the increased LV end-diastolic volume and eccentric hypertrophy act as compensatory mechanisms for long periods of time, which masks the development of afterload mismatch and the progressive exhaustion of myocardial contractile reserve.12
Therefore, more sensitive parameters of early LV dysfunction are needed (13). For detecting LV dysfunction, LV ejection fraction (LVEF) has been widely used. However, in chronic AR, because LVEF is preserved by increased preload, it cannot precisely detect the developing myocardial dysfunction (14). A more sensitive parameter of LV dysfunction than LV diameter and LVEF would be of considerable clinical value, as it would allow clinicians to detect early abnormalities, assist in the evaluation of symptoms, and indicate the need for vigilant observation, and possibly earlier surgery (15). Speckle-tracking strain analysis is a novel echocardiographic tool for an accurate tool to detect subclinical preoperative LV dysfunction.
Our work showed that the incidence of systolic dysfunction differs according to age, with higher incidence being in higher age groups, but it is not affected by gender. In our study, 25% of patients have developed systolic dysfunction and we found that the occurrence of postoperative LV systolic dysfunction not related to the etiology of severe AR. It has been shown that asymptomatic patients with chronic severe AR can have reduced systolic LV function while maintaining a normal global LVEF. This was explained by the fact that sub-endocardial fibers, which perform longitudinal LV contraction, are exposed to the increased wall stress at an early stage, and that this initiates a fibrotic process.16 This makes the strain parameters are useful to detect LV dysfunction at an earlier stage.17 This was concordant with Smedsrud et al.18 who demonstrated a reduction in GLS in 47 patients with severe AR compared with healthy control subjects (–17.5±3.1% Vs–22.1±1.8%, p<0.01), even in the presence of a preserved LVEF.18
Using ROC analysis, we found that the optimal cutoff value of preoperative GLS for prediction of postoperative LV systolic dysfunction is <-14% (80% sensitivity, 100% specificity, AUC =99%) while Olsen et al.19 found that in medically treated patients, a GLS of –18% was the best cutoff for the identification of disease progression while a cutoff of –14% was predictive of poor outcome in patients undergoing AVR which agreed with our results.19
After applying regression analysis, the present study showed that preoperative Global longitudinal strain (GLS) is the most significant independent variable in prediction of postoperative LV systolic dysfunction (p<0.001) also DF, EF but not E/e' are independent predictors. In this study, there is underlying systolic dysfunction which is detected by low preoperative GLS (-17.35±3.7) despite normal EF (60.35±7.21).
Preoperative EF has been regarded as a significant predictor for postoperative outcomes. Based on the previous reports, the EF was able to predict many adverse outcomes after surgical correction, such as the poor restoration of postoperative LV function, operative mortality (19). In our study, we found that there is a positive correlation between preoperative LVEF & postoperative LVEF, which is concordant with Onishi et al.20
In our study, there was a small, but significant, decrease in LVEF after AVR (60.35±7.21 vs 52.6±7.52). These results are concordant with those of Pomerantz et al.21 but differ from the results of others Bonow et al.,13 Borer et al.15 who showed an increase in LVEF after AVR. This apparent disagreement is due to our strict inclusion of patients with normal LVEFs. Increases in LVEF in patients with AR after surgery have been shown only in patients with reduced LVEFs before surgery.22 It was showed that EF >52% was the best cut-off value for successful early recovery of dilated LVEDD after AVR.23 This is close to our results which showed the best cutoff value of preoperative LVEF for prediction of postoperative LV systolic dysfunction (LVEF<50%) is <55% (80% sensitivity, 73.3% specificity, accuracy 75%, AUC=87%). Our results showed also that preoperative LVEF was proved inferior to GLS & DF in prediction of postoperative LV systolic dysfunction.
Up to our knowledge, No one thought to use the diastolic driving force "DF" as a predictor of outcome in patients with severe AR. In our study, DF proved to be better than E/e' to discriminate disease from normal, as with disease peak E changed more than peak A, but the ratio obscured the absolute differences from normal.24 DF equation was later validated for prediction of end diastolic pressure.7 The present study showed that there is highly significant negative correlation between DF and postoperative LVEF. ROC analysis yielded an optimal DF cutoff value of> 0.5newton for prediction of postoperative LV systolic dysfunction (sensitivity=80%, specificity 100%, accuracy=95%, AUC=89%). In the present work, DF has equal sensitivity (80%) but higher specificity than EF (100% VS 73.3% respectively), for prediction of postoperative LV systolic dysfunction. Compared to GLS, DF has equal sensitivity (80%) and specificity (100%) for prediction of postoperative LV systolic dysfunction. Regression analysis showed that DF is slightly inferior to GLS, but superior to EF in prediction of postoperative LV systolic dysfunction.
Preoperative GLS and DF seem to be independent predictors for postoperative LV systolic dysfunction after AVR for chronic severe AR. The optimal cutoff value of preoperative GLS is<-14% (80% sensitivity, 100% specificity, AUC=0.99), while that of DF is >0.5newton (80% sensitivity, 100% specificity, AUC=89%). Keeping in mind the limited availability of strain software in every echo machine, DF is an emerging, noninvasive, simple, and reliable preoperative parameter in predicting the outcome of AVR by adjusting the timing of surgery in chronic severe AR with normal EF.
Wide scale use of these two echocardiography parameters in patients with chronic severe AR to discover as early as possible the ideal timing of AVR.
None.
The authors declare that there is no conflict of interest.
None.

©2019 Gouda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.