Journal of
eISSN: 2373-4396


Review Article Volume 12 Issue 6
1Division of Cardiovascular Medicine, National University of Asuncion, Paraguay
2Department of Health Sciences Research, Metropolitan Sanatorium, Paraguay
Correspondence: Osmar Antonio Centurión, Professor of Medicine, Asuncion National University, Department of Health Sciences’s Investigation, Sanatorio Metropolitano, Teniente Ettiene 215 c/ Ruta Mariscal Estigarribia Fernando de la Mora, Paragua
Received: October 12, 2019 | Published: November 1, 2019
Citation: Centurión OA, Cáceres JD. Myocardial bridge as a cause of myocardial ischemia and infarction: “Bridge over troubled waters” and turbulent coronary blood flow. J Cardiol Curr Res. 2019;12(6):136-140. DOI: 10.15406/jccr.2019.12.00456
Myocardial bridge (MB) is a congenital coronary anomaly that is anatomically well recognized since the sixteenth century. Although, this condition is frequently asymptomatic, MB may be responsible for adverse ischemic-related complications including angina, myocardial ischemia, acute myocardial infarction, left ventricular dysfunction, cardiac arrhythmias, and even sudden cardiac death. The incidence of MB varies depending on the method of detection. It may vary from 1.5 to 16% when analyzed by angiography and up to 80% when assessed by autopsy. Flow alterations and turbulent coronary blood flow due to the MB can develop accelerated atherosclerosis in the coronary segment immediately proximal to the bridged segment. Other important factors to contemplate are the concomitant number of affected arteries or tunneled segments, and the degree of reduction in lumen diameter during systolic contraction or kinking. First-line therapy involves pharmacological treatment with beta-blockers and calcium-channel blockers, while nitrates are contraindicated due to adverse increase in heart rate and myocardial hypercontractility from reflex sympathetic activation. Non-pharmacological therapy, namely, surgical intervention and percutaneous coronary intervention with implantation of diluted stents should only be considered as a therapeutic option in patients with MB refractory to medical therapy and documented myocardial ischemia. A large, prospective randomized clinical trial is required to identify the best treatment strategy for patients with myocardial bridging.
Keywords: myocardial bridge, myocardial ischemia, myocardial infarction, coronary angiography, congenital coronary anomaly
The term myocardial bridge (MB) is utilized when the muscle tissue overlies the intra-myocardial segment of the epicardial coronary artery. MB is a congenital coronary anomaly that is anatomically well recognized since the sixteenth century.1 The prevalence of MB varies among different studies with different diagnostic methods. The rates reported in autopsy series range from 4.7% to 60%, and are much higher than those obtained in angiographic studies (0.5% to 12%). In addition, the prevalence rates increase to 40% when a positive inotropic medication is used as a provocative agent.2–4 Although, MB is frequently asymptomatic, it may produce ischemic-related signs and symptoms, including acute myocardial infarction, heart failure, ventricular arrhythmias, and even sudden cardiac death.5–11
MB was first observed by coronary angiography in 1960.12 Since then it was reported with other imaging modalities such as coronary computed tomographic angiography, intravascular ultrasound, optical coherence tomography, intracoronary Doppler, and fractional coronary flow reserve.13–17 These modern imaging techniques have enabled better analysis of the anatomic and hemodynamic consequences of the systolic compression by the MB, including pathological effects on coronary flow.17
Despite the increased comprehension of this condition, treatment options remain limited due to the lack of randomized clinical trials. Medical management with drug agents such as beta-blockers and calcium-channel blockers remain first-line therapy.18 Surgical excision of the MB is reserved only for refractory cases to pharmacological treatment.19,20 Percutaneous coronary intervention with stent implantation has been performed also in drug refractory patients. However, complications related to stent fracture,21,22 stent thrombosis, in-stent restenosis, and coronary perforations have been reported.23–26 It is our intention to describe the epidemiological and anatomical characteristics and analyze the clinical alterations produced by MB, and assess current treatment options including medical and invasive therapies.
In some cases there is more than one MB, either affecting the same coronary artery or different vessels.27–31 MB is traditionally considered a benign condition; however, its association with myocardial ischemia and infarction has considerably increased their clinical relevance. Anatomically, the muscle overlying the intramyocardial segment of the epicardial coronary artery is termed MB, and the coronary artery entering the muscle tissue is called tunneled artery. The coronary arteries may immerse deep into the myocardium for varying lengths, and then reemerged on the epicardial surface. Although MB can be found in any epicardial artery, most of them (up to 98%) compromise the LAD. The presence of the MB allows dividing the coronary artery into three segments: proximal, underneath and distal to the MB.28–30 The incidence of MB varies depending on the diagnostic auxiliary method utilized for detection. When this entity is analyzed by coronary angiography it may vary from 1.5 to 16%.30 However, when assessed by autopsy, its incidence may be elevated up to 80%.31 This dissimilarity in prevalence reflects the difference between an anatomical finding on autopsy, and the milking phenomenon on coronary angiography. The segments of coronary artery that penetrate the myocardial layers of the ventricle are surrounded by muscular fibers. Hence, these artery segments can greatly decrease in caliber during ventricular contraction in systole, an effect known as “milking”, due to the external pressure of the myocardial fibers on the coronary artery wall. Pathologic assessment of MB includes findings of thin bridges or even myocardial loops with little hemodynamic consequence than would not produce clearly visible systolic compression on angiography. These angiographically obtained rates can increase up to 40% if provocation tests are utilized (32). Coronary angiography remains the most common imaging technique for diagnosing myocardial bridging.
The coronary arteries are fed with blood flow mainly during diastole, and only 15% of coronary blood flow occurs during systole. Since the milking effect of the MB occurs in systole during ventricular contraction, the clinical relevance of MB has been challenged. Therefore, it appears unlikely that this systolic phenomenon could by itself result in ischemic-related complications. Nevertheless, we have to keep in mind that MB was associated to alterations in ventricular repolarization, myocardial ischemia and infarction, arrhythmias, and sudden cardiac death. These mentioned clinical changes and complications are related to myocardial ischemia produced by certain anatomical and physiological factors, namely, compression not only of the entrapped coronary artery segment, but also of the branches arising from or near the involved vessel segment.33–38 In addition, flow alterations, turbulent coronary blood flow, and axial stretch of the artery wall due to the MB can develop accelerated atherosclerosis in the coronary segment immediately proximal to the bridged segment. Other important factors to contemplate are the concomitant number of affected arteries or tunneled segments, and the degree of reduction in lumen diameter during systolic contraction or kinking.36–42
Clinical features and diagnostic management
The clinical signs and symptoms presenting in patients with MB are related to ischemia-related alterations. Therefore, clinical features may manifest as angina-like chest pain, coronary spasm, acute coronary syndromes, left ventricular dysfunction, ventricular arrhythmias, and even sudden cardiac death.42–49 Serious cardiac events are not common in the clinical setting. In addition, it is still arguable and uncertain whether MB can be directly attributable as the cause of the events. In studies involving patients with MB, myocardial ischemia was found in rates that varied from 21%–88%. This wide range is likely related to differences in test method sensitivity and specificity.50 It is interesting to note that myocardial ischemia was more closely associated with the degree of systolic compression rather than the location or length of the MB. The mechanism for ischemia is complex and is related to the delayed relaxation in the early diastole. It was observed that after the surgical release of the systolic occlusion by the MB, the diastolic flow increased proportionally with the duration of vessel occlusion, even though the increase in diastolic flow was not fully compensatory. As a consequence, the main diastolic flow and the coronary flow reserve are both reduced.49–54 The likelihood of ischemia depends on the location and longitude of the MB. Ischemia increases if MB is located more proximally in the coronary artery and the tunneled segment tends to be longer and/or deeper into the myocardium.51 In order to address prognostic implications, MB was classified into benign and pathological according to the intra- myocardial length and depth. The MB is therefore considered pathological when the tunneled segment is 20 to 30mm long, and 2 to 3 mm deep.53 Nevertheless, it has been demonstrated that ischemic alterations can also occur with shorter MB, demonstrating that benign MB can be clinically relevant and may have significant adverse effects.53–57 The MB passing over the tunneled coronary artery segment can cause narrowing of the artery during each systolic contraction. However, coronary flow is maximal during diastolic phase of the cardiac cycle and not during systole. Therefore, MB has long been considered as a normal anatomical variant without any hemodynamic or physiological relevance.54 However, ultrasound and coronary pressure wires evaluations demonstrated that during the period of highest coronary blood flow in early diastole, the MB is responsible for the delayed relaxation by reducing the distal coronary pressure.52–54
Therapeutic options in the management of MB
In the absence of randomized clinical trials comparing optimal medical treatment versus percutaneous coronary intervention (PCI) with drug-eluting stents, medical therapy seems to be the treatment of choice for most of the patients with MB. First-line therapy involves pharmacological treatment with beta-blockers and calcium-channel blockers, while nitrates are contraindicated due to adverse increase in heart rate and myocardial hypercontractility from reflex sympathetic activation. On the other hand, in those patients with documented ischemia or infarction, PCI with drug-eluting stents may be indicated for those severely symptomatic patients who are refractory to optimized medical treatment and who are not surgical candidates. It was demonstrated that PCI with stent implantation may improve hemodynamic abnormalities and relieve clinical symptoms.39 However, no studies have demonstrated yet normalization of myocardial perfusion defects after stent implantation. Moreover, there are certain concerns related to complications. It has been observed up to 6% of perforation during stent deployment.40–42 There are some reported cases of stent related complications, fracture and stent thrombosis.43–47 The percentage of in-stent restenosis is higher with bare-metal stents (up to 75% at 1 year) than with drug-eluting stents (up to 25% at 1year).46,47
The available data suggest that surgical therapy appears to be safe, beneficial and effective (Figures 1 & 2). Surgery may be recommended in the very rare cases of severely symptomatic patients who are refractory to medical therapy, or when PCI has failed.48,49 Non-pharmacological therapy, namely, surgical intervention and percutaneous coronary intervention should only be considered as a therapeutic option in patients with MB refractory to medical therapy and documented myocardial ischemia, with the expectation that revascularization rates will be high even with drug-eluting stents.
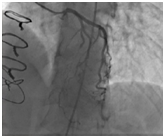
Figure 1 Snapshot of the preoperative coronary angiogram at systole showing compression of the proximal and mid-segments of the LAD. Reprinted with permission from Mok S, Majdalany D, Pettersson GB. Extensive unroofing of myocardial bridge: A case report and literature review. SAGE Open Medical Case Reports 2019;7:1-3.
Flow alterations and turbulent coronary blood flow due to the MB can develop accelerated atherosclerosis in the coronary segment immediately proximal to the bridged segment. Hemodynamic effects produced by the MB include systolic coronary flow reversal proximal to the bridge, as well as a decrease in coronary flow reserve. Ischemic-related clinical consequences range from symptoms of angina pectoris to acute coronary syndrome and sudden cardiac death. First-line therapy involves pharmacological treatment with beta- blockers and calcium-channel blockers, while nitrates are contraindicated due to adverse increase in heart rate and myocardial hypercontractility from reflex sympathetic activation. Non-pharmacological therapy, namely, surgical intervention and percutaneous coronary intervention with implantation of diluted stents should only be considered as a therapeutic option in patients with MB refractory to medical therapy and documented myocardial ischemia. A large, prospective randomized clinical trial is required to identify the best treatment strategy for patients with myocardial bridging.
None.
The authors declare that there are no conflicts of interest.
None.

©2019 Centurión, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.