Journal of
eISSN: 2373-4396


Case Report Volume 18 Issue 1
1Department of cardiology La Rabta teaching hospital, University of medicine Tunis, Tunisia
2Faculty of Medicine of Tunis, Tunis El Manar University, Tunisia
Correspondence: Mghaieth Zghal Fathia, Department of cardiology La Rabta teaching hospital, University of medicine, Tunis, Tunisia, Tel 0021698648676
Received: October 18, 2024 | Published: January 24, 2025
Citation: Fathia MZ, Asma B, Selim B, et al. Loffler endocarditis: When Hypereosinophilia is a route changer and echo is a pathfinder. A case report. J Cardiol Curr Res. 2025;18(1):1-4. DOI: 10.15406/jccr.2025.18.00616
Background and aim: Loeffler endocarditis is a rare complication of eosinophilic granulomatosis with polyangiitis. It is a heart infiltrative disease. Even if echocardiography is not the gold standard for tissue characterization, it can be important for an optimized diagnostic strategy.
This report aims to highlight imaging nuances that can be helpful for a better Loeffler endocarditis diagnosis
Case summary: This is the case of a Caucasian 67-year-old woman with a history of adult-onset asthma. She complained of increased dyspnea. Physical examination and electrocardiogram were non-specific. hypereosinophilia and immunological tests allowed the diagnosis of eosinophilic granulomatosis. Echocardiography showed a left ventricle apical mass with specific characteristics identified by conventional, 2D, and Doppler modes and further by Tissue Doppler and speckle tracking imaging. Cardiac magnetic resonance identified fibrosis centered by a thrombus. We retained the diagnosis of stage 2 to 3 Loeffler endocarditis, and corticoids and anticoagulants were used for treatment with a short-term favorable outcome and symptom relief.
Conclusion: In eosinophilic granulomatosis with polyangiitis patients, echocardiography should actively search for ventricular fibrosis, thrombi, myocardial dysfunction, and valve abnormalities. All of them are highly important and useful for Loeffler endocarditis at time diagnosis and management planning
Keywords: loeffler endocarditis, left ventricular thrombus, eosinophilic endocarditis, fibrosis, transthoracic echocardiography, cardiac magnetic resonance imaging, case report
CMR, Cardiac magnetic resonance; c-ANCA, cytoplasmic antineutrophil cytoplasmic; E, early diastolic pulsed wave velocity; E’, tissue doppler average early diastolic mitral annulus velocity; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; LV, left ventricle/ventricular; MPO, anti-myeloperoxidase; RV, right ventricle/ventricular
Loeffler endocarditis is a rare complication of hypereosinophilic syndrome (HES) secondary to heart infiltration by eosinophilic cells. W Loeffler first described it in 1936.1 This infiltration leads to endo-myocardial damage and activates coagulation and fibrosis processes. It also impairs relaxation and decreases the diastolic function of the heart. The gold standard diagnostic test is endomyocardial biopsy.2 Cardiac magnetic resonance imaging (CMR) is increasingly used as a non-invasive diagnostic tool.3 Echocardiography remains a major first-line imaging powerful tool for lesion detection and functional left and right ventricle (LV and RV) assessment. This case emphasizes the contribution of ultrasounds to the diagnostic pathway of Loeffler endocarditis secondary to eosinophilic granulomatosis and polyangiitis (EGPA).
This is the case of a Caucasian 67-year-old female farmer with no family history of chronic disease, heart disease, or sudden death and a personal history of adult-onset asthma and peripheral sensitive neuropathy. She complained of progressive dyspnea grade II on the NYHA scale that started 2 months ago, associated with recent asthenia. There was no decubitus dyspnea or coughing. The patient did not declare having a fever, chest pain, palpitations, or lower limb swelling.
On physical examination blood pressure was symmetrical at 110/70 mmHg, heart rhythm was 65/minute, respiratory rate was 20/ minute, she had no cyanosis nor pallor, and peripheral oxygen saturation was 99%. We noticed a 4th heart sound without a heart murmur and bilateral wheezing on pulmonary auscultation without crackles. Arterial axes palpation and auscultation were normal, there was no skin abnormality. We did not find liver enlargement and the hepatic surface was smooth.
The electrocardiogram showed a regular sinus rhythm, LV systolic stress (negative T waves in V5-V6 derivations), and no sign of LV hypertrophy (Sokolow index was 34 and Cornell index was 18). Conduction (PR, QT) intervals were normal.
Laboratory tests showed eosinophilic hyperleukocytosis (white cells:10460/mm3; Normal range (NR) [4000-1000/mm3] , eosinophils:5530/mm3; [0-500/mm3] ), and inflammation (C reactive protein: 60 mg/l; NR [<10 mg/l]). B natriuretic peptide was 147pg/ml; NR [<100 pg/ml]. The other parameters were normal Hemoglobin:12 g/dL; NR [12-15 g/dl], platelets:321000/mm3; NR [150000-450000/mm3] hypersensitive troponins 0.012ng/ml; NR [<0.01 ng/ml], creatinine level was 0.8 mg/dl; NR [0.5-1.1 mg/dl], and no electrolyte disturbance was noticed.
The Immunological ELISA tests were positive for cytoplasmic antineutrophil cytoplasmic (c-ANCA) and anti-myeloperoxidase (MPO) antibodies.
Chest X-ray showed a normal cardiac silhouette and no signs of pulmonary congestion. It revealed however bilateral bronchial wall thickening and vertebral scoliosis.
Transthoracic echocardiography
The four-chamber apical view (Figure 1A, 1B) showed preserved contraction with an echoic apical structure adhering to the wall without evident trabeculations or recesses. LV was neither dilated nor hypertrophied, the apex was hypokinetic. The atria were not dilated. There was no epicardial effusion and valves were normal.
To better characterize this apical obliteration, we used color Doppler while reducing the speed scale to detect low velocities, but it was not penetrated by color (Figure 1C).
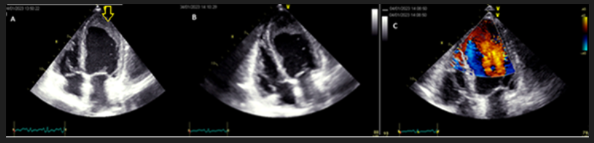
Figure 1 Apical four-chamber views
A: Apical bidimensional four-chamber view; apical filling with an echoic structure (yellow arrow)
B: Focused apical four-chamber view; no morphological right ventricle abnormality
C: Four chamber view Color Doppler with speed rate scale reduction; no color penetration into the apical structure
Segmental kinetic analysis showed hypokinesis of the apex, tissue Doppler (TDI) showed cyclic deformation of the mass that did not follow systole or diastole times (figure 2A), this finding was confirmed by speckle tracking longitudinal 2D strain imaging as well (Figure 2B).
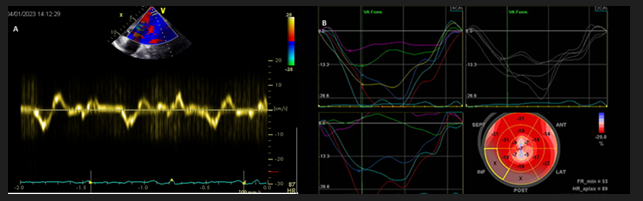
Figure 2 Myocardial mechanics analysis by tissue Doppler and bidimensional speckle tracking strain
A: Tissue Doppler pulsed wave curve in the apical structure; deformation that did not follow electrical systolic and diastolic times.
B: 2D strain curves and bull-eye, apical strain reduced velocities and anarchic deformation
Regarding diastolic function, we found a prolonged LV isovolumic time 170 ms; NR [60-110 ms] indicating an impaired relaxation, a type 2 pseudo normal mitral profile with an early diastolic pulsed wave velocity (E) at 67cm/s. Tissue Doppler average early diastolic mitral annulus velocity (E’) was reduced (6cm/s). Giving mitral profile was pseudo normal we used the three following criteria to assess LV filling pressures witch were negative: E/E’=11 [<14], tricuspid regurgitation velocity was 2.4m/s [<2.8m/s], and the indexed to bady surface left atrial volume 29ml:m2 [<34ml/m2]. Thus we concluded to a moderate relaxation dysfunction without LV filling pressures elevation.
Then we focused on the right ventricle (RV) which was neither hypertrophied nor enlarged and did not show exaggerated trabeculation nor thrombus (Figure 1B).
In summary, we found an apical structure adhering to the LV wall, not penetrated by color Doppler, with a proper deformation that did not follow another myocardial deformation cycle. RV was structurally and functionally normal.
Cardiovascular magnetic resonance
Cine sequences (Figure 3A) showed an apical filling centered by a hypodense image suggesting a thrombus. Ten minutes after gadolinium injection (Figure 3B), the mass had a late enhancement, in favor of fibrosis, centered by a non-enhanced image which was a thrombus.
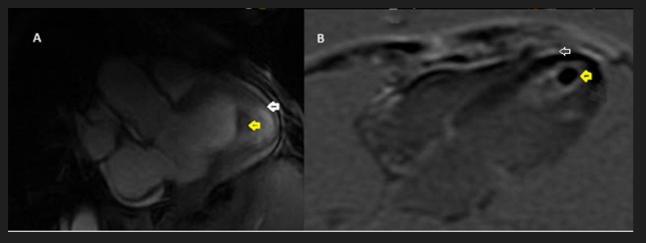
Figure 3 Cardiac magnetic resonance imaging
A: Cine sequence 3 chamber view; apical filling (white arrow) with a central black structure (yellow arrow).
B: Delayed post Gadolinium injection sequence; apical enhanced fibrosis (white arrow) centered by a non-enhanced thrombus (yellow arrow)
Diagnosis and therapeutic management
we retained the diagnosis of EGPA in this patient based on the association of mixed vasculitis and eosinophilic clinical and laboratory signs; asthma (+3), polyneuritis (+1), inflammation, hypereosinophilia (+5), and anti-Anca and anti-MPO antibodies (-3) in a farming favorable ground of farm worker. when applying the American college of Rheumatology (ACR) and European Alliance of Associations of Rheumatology (EULAR) 2022 criteria for the classification of vasculitis cohorts, the total score is 6 which is the lowest threshold needed for EGPA . In addition, Stage 2 (thrombotic) to 3 (fibrotic) left-sided Loeffler endocarditis was diagnosed, presenting as cardiac involvement in severe eosinophilic granulomatosis with polyangiitis.
The patient received anticoagulants (Subcutaneous Enoxaparin switched to oral Acenocoumarin) and corticosteroids. There was no in-hospital clinical event and at 3 3-month visits the patient was asymptomatic with relief of her dyspnea, PE did not show heart failure or pulmonary or hemorrhagic signs.
This is a case of EGPA-associated LV Loeffler's endocarditis, the diagnosis was oriented by clinical history and routine biology including late-onset asthma, polyneuritis, and hypereosinophilia. Reciprocally, these elements should be carefully analyzed in the etiological investigation of cardiomyopathy. We focused on ultrasound description, as echocardiography is the first-line imaging key tool. Even final confirmation should rely on CMR or myocardial biopsy.
Loeffler’s endocarditis occurs in 40 to 50% of HES patients.4 HES has many etiologies. In our patient, it was related to EGPA (formerly known as Churg-Strauss disease). She scored 6 in ACR/EULAR 2022 classification5 used for vasculitis research cohorts. Cardiac involvement classifies EGPA as severe.6 It is, indeed, a main prognostic indicator; responsible for frequent intensive care admissions and one-third of deaths.7,8
It is noteworthy that this patient with c-ANCA and anti-MCO positive antibodies (which is the immunological finding in 40% of EGPA patients) shows an overlap between vasculitis phenotype (that includes purpura glomerulonephritis or polyneuritis in this case) related to ANCA positive profile and hypereosinophilia phenotype including cardiac involvement which is rather related to ANCA negative profile.9
Eosinophil degranulation releases toxic ribonucleases, major basic proteins, and oxygen-reactive species, leading to endomyocardial necrosis and apoptosis. Eosinophilic infiltration leads to the inflammatory, often subclinical, first stage.10 A procoagulant state induced by the same released cationic proteins (binding endothelial thrombomodulin binding and coagulation regulatory complexes inhibition) added to wall lesions, triggers platelet activation and intravascular/intracavitary thrombi formation, that characterizes the second stage. In the third stage, fibrosis progressively substitutes thrombi and infiltrates endomyocardial damaged tissue, it can extend to heart valves and conduction fibers.4,11 This stage is often interchangeably referred to as endomyocardial fibrosis. Our patient was in stages 2 to 3.
Cardiac manifestations depend on the disease stage, they typically include LV dysfunction, restrictive cardiomyopathy, pericardial disease (acute pericarditis of constriction), valve insufficiency, and thromboembolic events. They also can consist of ventricular or supraventricular conduction abnormalities and ischemic heart disease due to hypereosinophilic coronary inflammation.12
In most cases Loeffler endocarditis is biventricular, but it can affect only one ventricle, being left-sided more often.13 In our case RV appeared normal in morphology and function this does not exclude the possibility of histological lesions.
Multimodal cardiac imaging is recommended based primarily on echocardiography and CMR. We emphasized how to optimize echocardiography to help diagnosis. Apical obliteration is a key finding, but it has many differential diagnoses; apical hypertrophy, hyper trabeculation, tumor…etc). In addition to the conventional bidimensional imaging, we used the color Doppler and deformation imaging with both TDI and 2S strain. Echocardiography could not however distinguish the heterogeneity of the apical filling with fibrosis. Shah et al reported that contrast echocardiography was superior to non-enhanced echocardiography in identifying and delimitating apical obliteration.14 CMR had a higher accuracy. Late enhancement was important for fibrosis identification. In earlier stages, late enhancement and T2 sequences could detect edema and inflammation. In total, echocardiography is crucial for diagnostic orientation and comprehensive cardiac assessment of chamber morphology, systolic and diastolic functions, and valve or pericardial disease.15 CMR allows confirmation removing the need to go to myocardial biopsy.16
Relying on current recommendations,9 in such case of neuropathy and heart involvement, an intensive immunosuppression with high-dose corticosteroids and cyclophosphamide is indicated (or rituximab), but we considered the infectious pulmonary risk and used only standard dose oral corticosteroids. Anticoagulation is effective in reducing thromboembolic risk. Mepolizumab; a monoclonal antibody that reduces eosinophils, is used in the maintenance phase. However, it was not available in our context.
This case illustrates practical effective diagnostic steps and the central role of echocardiography in avoiding diagnostic delays and then prognosis worsening. We recognize some limits, indeed the presented findings could not be applicable in earlier stages, on the other hand, we did not use useful techniques like contrast echocardiography.
Loeffler endocarditis is a rare progressive disease from subclinical to restrictive cardiomyopathy and terminal heart failure. The earlier the diagnosis, the better the reversibility of lesions and the prognosis. Echocardiography use should be optimized to orient the diagnosis. CMR has a high accuracy. Multidisciplinary management of this systemic disease is essential.
The patient gave her informed written consent to participate
The patient gave her informed written consent for publication
All data are available for consultation upon requested
All authors declare not to have any competing interests for this publication
This publication is not supported by any kind of funding
Dr. Mghaieth Zghal Fathia was the senior echocardiographer who made the echo exploration and the main author of this paper. Dr Brahim Asma, wrote the first draft and the clinical presentation while ensuring patient’s care. Dr Boudiche Slim contributed to the patient’s care and follow-up and revised the manuscript. Dr Ben Halima contributed to the manuscript validation and English and scientific revision. Dr Jebberi Zeynab managed the patient at her first presentation and performed wide bibliographic research and summarized articles to use them in the discussion part. Dr Zaouia Khaled helped in bibliographic research and figure editing. Dr Mourali Sami revised the finished paper and gave valuable remarks for its improvement.
To all health team contributors to the patient’s care and the patient and her family for their high quality of cooperation and positive interaction with the medical team.

©2025 Fathia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.