Journal of
eISSN: 2373-4396


Review Article Volume 3 Issue 2
1Department of Medicine, University Hospitals Case Medical Center, USA
2Cardiovascular Research Institute and Department of Medicine, Case Western Reserve University School of Medicine, USA
3Institute for Transformative Molecular Medicine and Department of Medicine, Case Western Reserve University School of Medicine, USA
Correspondence: Puneet Anand, Institute for Transformative Molecular Medicine, Case Western Reserve University School of Medicine, Wolstein Research Building 4107, 2103 Cornell Road, Cleveland, OH 44106 USA
Received: June 26, 2015 | Published: July 27, 2015
Citation: Matto F, Anand P, Zhang R, et al. Current and future therapies for management of systolic heart failure. J Cardiol Curr Res. 2015;3(2):1-6. DOI: 10.15406/jccr.2015.03.00093
Heart failure is a complex pathological condition characterized by the reduction in the ability of heart to pump blood to peripheral organs. There has been considerable advancement in the management of heart failure, and the disease modifying therapeutic options available currently have managed to enhance survival of HF patients. Use of advanced therapies and mechanical devices as a bridge to transplant (or even as definitive therapy for end-stage failure) is a standard practice. However, the mechanisms driving progression of heart failure is not well understood, and there is increasing realization that traditional models have been inadequate in explaining the progression of heart failure. Therefore, considerable interest has been generated to search for alternate models that might better explain and/or offer more targeted disease modifying agents. In this review, we discuss the currently available options for the management of systolic heart failure and new promising targets that may advance the paradigm of heart failure therapeutics.
Keywords:heart failure, mechanisms, management, therapeutic options
HF, heart failure; EF, ejection fraction; CAD, coronary artery disease; RAS, renin angiotensin system; eNOS, endothelial nitric oxide synthase; ICD’s, implantable cardioverter-defibrillators; CRT, cardiac-resynchronization therapy; LVAD, left ventricular assist devices
Heart failure (HF) is a pathological condition that impairs the ability of the heart to keep up with the metabolic demands of the body, resulting in multi-organ abnormalities and eventually death.1,2 Reduced ejection fraction (EF) is usually defined as EF<45-50%. This can result from conditions that reduce the ability of heart to pump blood which include decreased contractility of myocardium secondary to reduced coronary perfusion, structural causes like damaged heart valves, cardiac muscle or pericardial diseases etc.3 The mechanistic details that drive the progression of heart failure is not well understood but molecular and cellular changes point towards the role of neuro-hormonal activation and ventricular remodeling as primary determinants.4 The sequential and progressive upregulation of renin-angiotensin aldosterone system, increased levels of norepinephrine and endothelin-1 play a significant role in the progression of LV dysfunction. Coronary artery disease (CAD), Diabetes, and Hypertension are among the leading causes whereas other factors such as genetic, alcohol abuse, infectious myocarditis, chemotherapeutic agents etc also account for significant number of cases.5,6 The symptoms of decompensated HF are very non-specific (shortness of breath, fatigue, weakness, etc) and need evaluation in the context of the overall clinical picture, including signs like extremity edema, jugular venous distention, S3).5,7 Tests like BNP, ECG, CXR, echocardiography, C-MRI allow confirmation of the diagnosis.5
HF affects about five million people in the US alone. The numbers are staggering with close to fifty percent mortality within five years of diagnosis.8 It is the leading cause of hospitalization, and healthcare expenditures in the US with the total direct and indirect costs as high as $32billion each year,9 which is estimated to increase to $70billion by 2030.10 In fact, by 2030 an estimated 8 million people will suffer from HF in the US with direct healthcare costs of $53 billion, an increase of almost 150% from 2010.10 Thus, there is an urgent need for the development of new therapeutics for the treatment of heart failure.
Various models have been proposed to explain the mechanisms that precipitate and drive the progression of heart failure. The Renin Angiotensin system (RAS) dependent, retention of excessive salt and water (cardio-renal model) 11 or abnormal pumping capacity of the heart (hemodynamic model) have traditionally been the models proposed. There is usually an initial event that damages the heart muscle with resultant loss of functioning myocytes that causes decline in the pumping capacity of the heart. This decline leads to activation of a number of compensatory mechanisms including activation of the sympathetic system and the RAS.11,12 Increase in the sympathetic tone causes elevation in circulating levels of norepinephrine, leading to increase in ionotropy and chronotropy as well as peripheral vasoconstriction (Figure 1).11 The activation of these mechanisms does delay the onset of symptoms; however by augmenting myocardial oxygen demand, this can intensify ischemia and increase propensity for arrhythmias. Increased levels of angiotensin produced by RAS activation promote vasoconstriction and increases salt and water retention by increasing aldosterone levels.11 These factors in addition to blunted responsiveness of kidneys to natriuretic peptides (ANP, BNP) lead to worsening of fluid retention.12,13 In addition eNOS (endothelial nitric oxide synthase) mediated vasodilation in HF patients is blunted, which is secondary to attenuated eNOS activity.14,15 The sustained activation of neurohormonal and cytokine systems cause a series of changes in the myocardium that have been collectively referred to as remodeling and develop generally when patients transition to symptomatic HF. Inducible NOS (iNOS) (which shows increased expression in advanced HF patients15) may influence pathological remodeling as transgenic mice deficient in iNOS were found to have improved survival and remodeling after an ischemic event.16,17
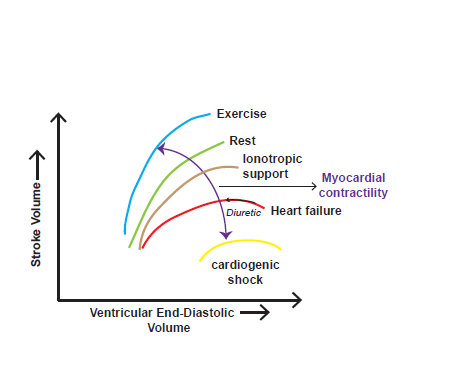
Figure 1 Depiction of the interrelation between Stroke Volume and Ventricular End-Diastolic Volume (Frank Starling curves). The curves show differences in ventricular performance between a normal resting heart, during exercise, in heart failure, and with ionotropic support in heart failure. Higher levels of EDV, which are associated with higher filling pressures, result in pulmonary edema and dyspnea and by lowering EDV, diuretics help in relieving symptoms.
Although lifestyle modifications have been routinely recommended for heart disease, these interventions have not been studied in clinical trials and are based on clinician experience and routine practice. Some evidence has been put forward that aerobic exercise might benefit heart failure patients by improving the quality of life and reduce hospitalizations. In addition certain dietary therapies, such as a low sodium diet that are prescribed for heart failure patients are based on slim evidence 18. Chronic HF is managed by administering various medications that help increase the cardiac function by lowering the pressure overload (neuro-hormone blockers), and decrease fluid retention (diuretics). Diuretics provide symptomatic relief by relieving fluid retention, with loop diuretics being the first choice in all but a few patients. Combination of loop and thiazide diuretic (in addition to an aldosterone antagonist that produces what is called sequential nephron blockade) is usually required in case of severe heart failure. Diuretic therapy may provide symptom relief but these agents have not been shown to provide mortality benefit.
ACE inhibitors
ACEIs are the first line therapy for heart failure with reduced EF. These agents prevent remodeling in addition to their effect on blood pressure and after load reduction. In patients with left ventricular systolic dysfunction, treatment with ACEIs is recommended. In clinical trials carried out with patients who have NYHA class II, III or IV HF, enalapril was shown to provide mortality benefit with relative risk reduction in death due to HF.19˗21 Enalapril was also shown to have mortality benefit over the combination of hydralazine and isosorbide dinitrate.22,23 ARB’s have also been shown to be equally efficacious as ACEI in the treatment of HF and appear to provide the same mortality benefit as ACEIs.24 In addition, these agents can be used as additional therapy for patients whose symptoms persist on optimal therapy. RCT’s involving ARBs studied role of valsartan and candesartan in reducing cardiovascular mortality as compared to placebo and were found to decrease readmission rate by 17-22% and reduce mortality by 16%.25
Beta-blockers
Beta-blockers are the mainstay of initial treatment for heart failure with systolic dysfunction as these agents are very important disease modifying drugs. By reducing metabolic demand, beta-blockers cause reduction in symptoms and improve systolic function with resultant increase in EF. Beta-blocker therapy in RCT’s was found to reduce mortality by 34% in conjunction with optimal HF therapy.26 Only bisoprolol, carvedilol and metoprolol succinate have been extensively studied and shown to have mortality benefits in all classes of heart failure.26˗30 In NYHA class III & IV, aldosterone antagonists have been shown to have survival benefit,31 particularly in patients who remain symptomatic despite treatment with diuretics, ACEI, and beta-blocker. In black patients, trials comparing the use of enalapril and the combination of hydralazine and isosorbide dinitrate showed that the patients responded much better to the combinatorial therapy.
Mechanical devices
Ventricular arrhythmias are the leading cause of death in patients with systolic heart failure, and implantable cardioverter-defibrillators (ICD’s) have been shown to reduce the risk of sudden death in patients with severe LV systolic dysfunction.32,33 Patients with class II or III HF with EF ≤35% who have life expectancy ≥ 1year have indication for ICD as primary prevention and in patients who survive an unprovoked episode of ventricular fibrillation or sustained ventricular tachycardia as secondary prevention.32 In patients who meet the following criteria--Class III-IV heart failure, ejection fraction ≤35% and QRS duration of 120 ms or more--cardiac-resynchronization therapy (CRT) has been shown to be beneficial.34,35 The synchronization of atrial and biventricular pacing improved cardiac function and resultant symptom improvement and functional capacity.35˗37 This was demonstrated in a retrospective analysis of patients with class IV symptoms, showing an increase of 45 m in the 6minute walk distance, a 25 point improvement in the Minnesota Living with Heart Failure score, and reduction by at least one NYHA functional class in 78% of the patients. Outcomes in advanced systolic heart failure have improved with the use of medical and device therapies (as discussed above) but eventual progression of the disease requires pursuance of more radical approaches. Ionotropic and advanced therapies may support the clinical status in the short term but long term survival is difficult to achieve on these therapies.38,39 The advent of newer continuous flow Left Ventricular Assist Devices (LVAD) have made these devices a more viable options as a bridge to transplantation.40,41 Older pulsatile devices were associated with more incidence of infections, bleeding, stroke, and device failures that required repairing or replacing the device.42 Continuous flow devices have been proven to be more effective as compared to older devices and have been shown in trials to improve survival to the extent newer devices are being offered as destination therapies.43 In a trial comparing continuous flow devices vs older pulsatile devices, newer devices were found to significantly improve probability of survival from stroke and device failure at 2 years in advanced heart failure patients.40
In patients who are considered refractory to therapy, heart transplantation is usually the last resort.44 Given the difficulty in obtaining suitable donor organs, patients have to meet very specific criteria, and they also should be able to withstand surgery in addition to intensive medical therapies after the transplant.45,46
New mechanisms involved in heart failure
Even though the current medications have dramatically helped improve the condition of the HF patients, the current high mortality rate and the lack of any new drugs in the last two decades has necessitated the development of new HF drugs. The neuro-humoral model might explain some aspects of the disease progression seen in the failing heart, but other models have been put forth that can explain some of the mechanistic details that drive pathological remodeling of the myocardium.4 There have been studies that have proposed promising pathways that can be exploited as new therapies for treating HF. Members of the bromodomain and extraterminal (BET), a family of epigenetic reader proteins, have been shown to be a critical mediator of pathologic cardiac hypertrophy and HF pathogenesis.47,48 In a recent study, the authors used a well-known specific inhibitor of BET, called JQ1, in the mouse model of pressure overload and they found out that the targets of BET overlap with the pattern in human diseased hearts, supporting the notion that BET inhibition may subserve a novel therapeutic approach in heart failure.47 In another recent study, it was demonstrated that mice with chronic PDE9A (phosphodiesterase 9A) inhibition exhibited reversal of pre-established hypertrophy in a nitric oxide-independent manner.49 The same study also showed an upregulated expression of PDE9A not only in left ventricular hypertrophy in humans but also in heart failure with preserved ejection fraction (HFpEF).49 Therefore, developing a therapy targeted targeting PDE9A inhibition may be considered a promising treatment for HF patients. Collectively, these novel findings clearly point towards exploration of new therapeutic approaches for heart failure.
Recent FDA approvals for heart failure drugs
Recent approval of more HF drugs by FDA is promising. LCZ696, a combination of two blood pressure lowering drugs valsartan and sacubitril, is an investigational drug that can help reduce the stress on the diseased heart.50 It is recommended for the treatment of heart failure with reduced ejection fraction (HFrEF). FDA has granted a priority review to LCZ696 which could expedite the access of the medicine to the patients. Also, ivabradine (which selectively inhibits the pacemaker lf current) was approved by FDA for patients with the higher risk of hospitalization due to chronic worsening HF,51 it reduced hospitalization for worsening heart failure or cardiovascular death by 18%52 and significantly reduced the risk of death from heart failure by 26% and hospitalization for heart failure by 26%.52
Diverse underlying mechanisms for HF and other complications such as diabetes, obesity and lifestyle make developing effective therapies challenging. However, constant discoveries of novel pathways and drug development have increased our understanding about both the pathogenesis of the disease and the pathways to be exploited.
None.
Author declares there are no conflicts of interest.
None.

©2015 Matto, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.